Question
Question: In order to determine the value of \({{E}_{0}}\), a scientist shines photons (“light particles”) of ...
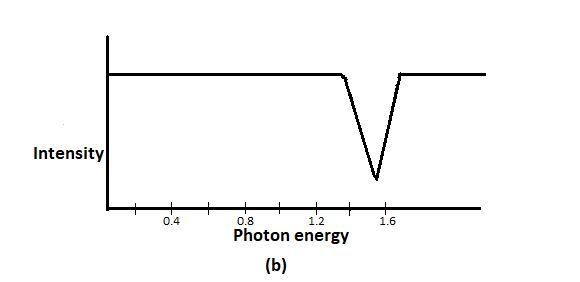
In order to determine the value of E0, a scientist shines photons (“light particles”) of various energies at a cloud of atomic hydrogen. Most of the hydrogen atoms occupy the ground state. A detector records the intensity of light transmitted through that cloud; as shown in the figure above. The graph in the above figure is that of part of the scientist’s data, showing the intensity of the transmitted light as a function of the photon energy. A hydrogen atom electron is likely to absorb a photon only if the photon gives the electron enough energy to knock it into a higher shell.
According to this experiment, what is the approximate value of E0?
A.1.6×10−18J
B.2.1×10−18J
C.3.2×10−18J
D.6.4×10−18J
Solution
In this question, we find the approximate value of E0 according to the experiment. We use Bohr’s model for solving this question. We find the energy on the first and second excitation energy. Then take the difference between them to find out the value of E0.
Complete answer:
Given:
Photon energy =1.6×10−18J
From figure b photon energy 1.6×10−18J gets absorbed in large numbers, no lower energy photon gets absorbed, and according to the passage, substantial absorption occurs only if the photon jumps the ground-state electron into a higher shell.
So, 1.6×10−18J photon knocks a ground-state electron (n=1) into the first excited state (n=2). So, the difference in energy between the ground and the first excited state must be 1.6×10−18J.
Using the below equation
En=n2E0
Now we find the energy for E1
E1=12E0
Now we find the energy for E2
E2=22E0
Now we substitute E1 from E2 then we get,
1.6×10−18=E2−E1
We have the values of the E1 and E2 so we put these values in the below equation
1.6×10−18=−4E0−(1−E0)
After simplifying this equation we get,
1.6×10−18=43E0
Then,
E0=34(1.6×10−18)
After solving this we get,
E0=2.1×10−18J
Here is the value of the E0 according to the experiment.
So option B is correct.
Note:
To solve this question students know the energy states and Bohr's model. Bohr model depicts an atom as a small, positively charged nucleus surrounded by electrons. These electrons travel in circular orbits around the nucleus, similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction. If an atom, ion, or molecule is at the lowest possible energy level, it and its electrons are said to be in the ground state. If it is at a higher energy level, it is said to be excited, or any electrons that have higher energy than the ground state are excited. After learning about these terms you can easily solve this type of question.
