Question
Question: In nature, many electron transfer reactions take place which are catalyzed by enzymes. Two such half...
In nature, many electron transfer reactions take place which are catalyzed by enzymes. Two such half-cell reactions are:
2H+(aq)+2e−⟶H2 (g, 1 atm)
O2 (g, 1 atm)+4H+(aq)+4e−⟶2H2O (l)Eo=1.23 V
The reduction potentials for the two half-cell reactions respectively at the physiological pH 7 are:
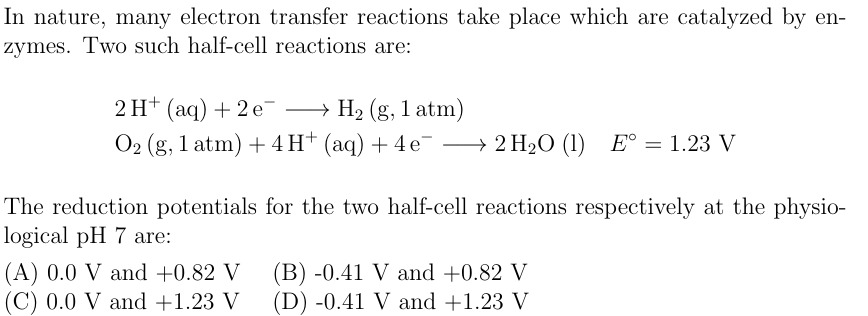
0.0 V and +0.82 V
-0.41 V and +0.82 V
0.0 V and +1.23 V
-0.41 V and +1.23 V
The reduction potentials for the two half-cell reactions at physiological pH 7 are approximately -0.41 V and +0.82 V, respectively.
Solution
To find the reduction potentials at physiological pH 7, we use the Nernst equation:
E=Eo−n0.0591logQ
where Eo is the standard reduction potential, n is the number of electrons transferred, and Q is the reaction quotient. At pH 7, the hydrogen ion concentration [H+] is 10−7 M.
For the first half-cell reaction:
2H+(aq)+2e−⟶H2 (g, 1 atm)
-
Standard Reduction Potential (Eo): By definition, the standard reduction potential for the Standard Hydrogen Electrode (SHE) is Eo=0.0 V.
-
Number of electrons (n): From the balanced reaction, n=2.
-
Reaction Quotient (Q): Q=[H+]2PH2
Given PH2=1 atm and [H+]=10−7 M.
Q=(10−7)21=10−141=1014
-
Calculate E at pH 7:
E1=Eo−n0.0591logQ
E1=0.0−20.0591log(1014)
E1=0.0−20.0591×14
E1=0.0−0.0591×7
E1=−0.4137 V≈−0.41 V
For the second half-cell reaction:
O2 (g, 1 atm)+4H+(aq)+4e−⟶2H2O (l)
-
Standard Reduction Potential (Eo): Given Eo=1.23 V.
-
Number of electrons (n): From the balanced reaction, n=4.
-
Reaction Quotient (Q): Q=PO2[H+]41 (activity of pure liquid water is 1)
Given PO2=1 atm and [H+]=10−7 M.
Q=1×(10−7)41=10−281=1028
-
Calculate E at pH 7:
E2=Eo−n0.0591logQ
E2=1.23−40.0591log(1028)
E2=1.23−40.0591×28
E2=1.23−0.0591×7
E2=1.23−0.4137 V
E2=0.8163 V≈+0.82 V
Thus, the reduction potentials for the two half-cell reactions at physiological pH 7 are approximately -0.41 V and +0.82 V, respectively.
