Question
Question: In \({K_2}C{r_2}{O_7}\), every \(Cr\) is linked to: A.Two oxygen atoms B.Three oxygen atoms C....
In K2Cr2O7, every Cr is linked to:
A.Two oxygen atoms
B.Three oxygen atoms
C.Four oxygen atoms
D.Five oxygen atoms
Solution
Potassium dichromate consists of dichromate ion and has a chemical formula Cr2O72−. To answer this question, you must recall the structure if the dichromate ion. Each chromium has 6 bonds.
Complete answer:
The name dichromate has a prefix di-, suggesting that the ion contains two chromate ions joined together.
The atomic number of chromium is 24 and its electronic configuration can be written as:
Cr:[Ar]4s13d5
Thus, a chromium atom can form 6 covalent bonds.
There is one chromium atom bonded to four oxygen atoms. Two oxygen atoms are double bonded with the chromium while the other 2 form single bonds. These two oxygen atoms satisfy their valencies with an extra electron denoted by the charge on the ion. The structure of chromate ion can be drawn as
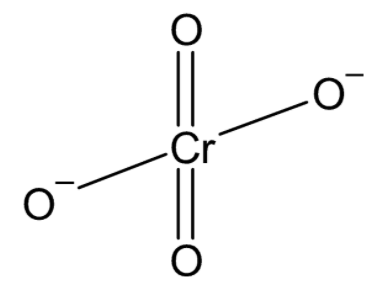
In the dichromate ion, there are two chromium atoms bonded to a total of seven oxygen atoms. One oxygen atom acts as a bridge bonded to each chromium atom. Two oxygen atoms on each chromium atom are double bonded to chromium and one oxygen on each chromium carries a negative charge giving the ion a total charge of −2. The structure of the dichromate ion can be drawn as:
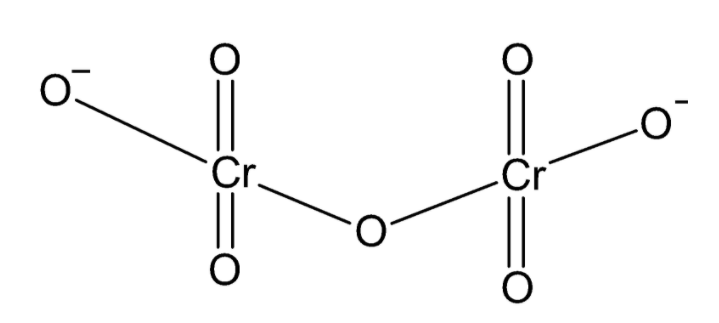
From the structure of the ion, we can conclude that each chromium atom is bonded to four oxygen atoms.
Thus, the correct answer is C.
Note:
We can see that two tetrahedral shaped chromate units share a common oxygen atom forming dichromate ion. So, the number of oxygen atoms bonded to chromium atoms remains the same, i.e., four in both chromate and dichromate ions.
