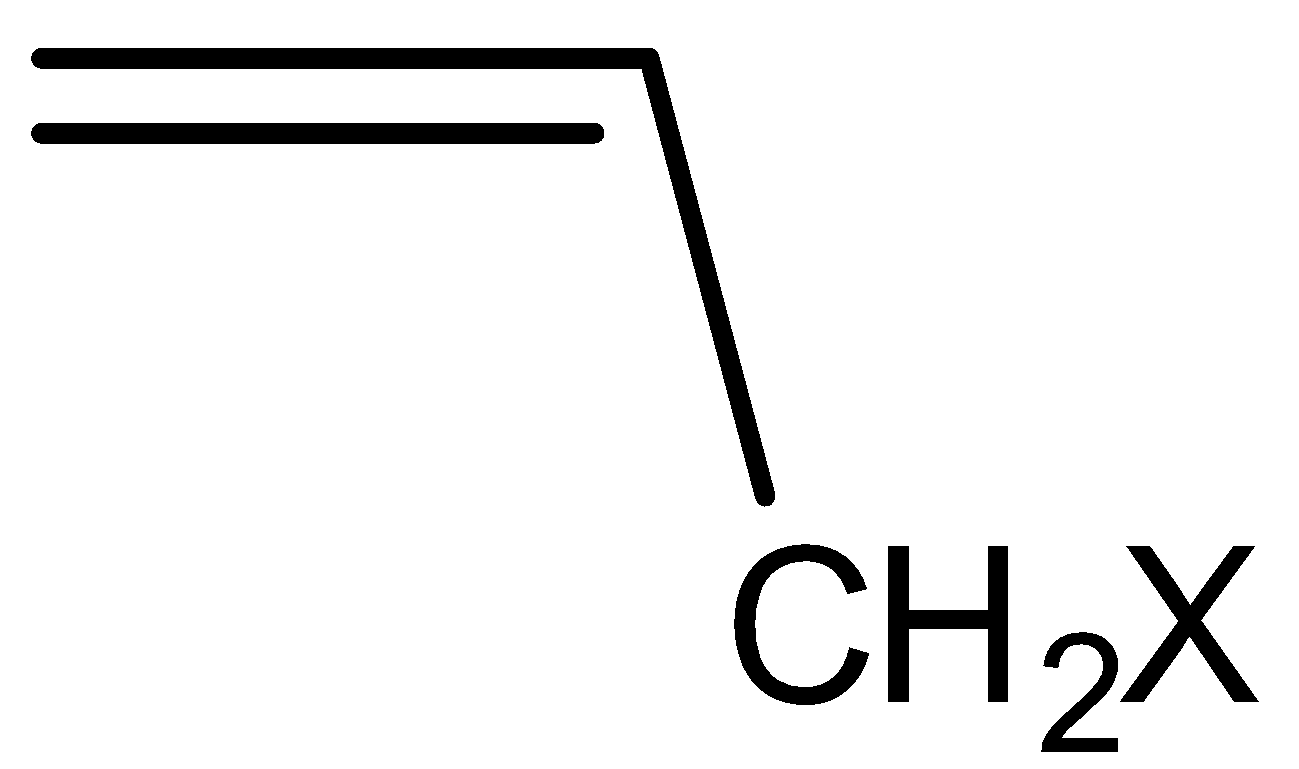
Question
Question: In its nucleophilic substitution reaction, aryl halide resembles A.Vinyl chloride B.Allyl chlori...
In its nucleophilic substitution reaction, aryl halide resembles
A.Vinyl chloride
B.Allyl chloride
C.Benzyl chloride
Solution
We know that halides are classified as sp2C−X and sp3C−X. sp3C−X type of halides are further classified into three types namely, alkyl halides, allylic halides and benzylic halides. sp2C−Xtypes of halides are classified into two types namely, vinylic and aryl halides.
Complete step by step answer:
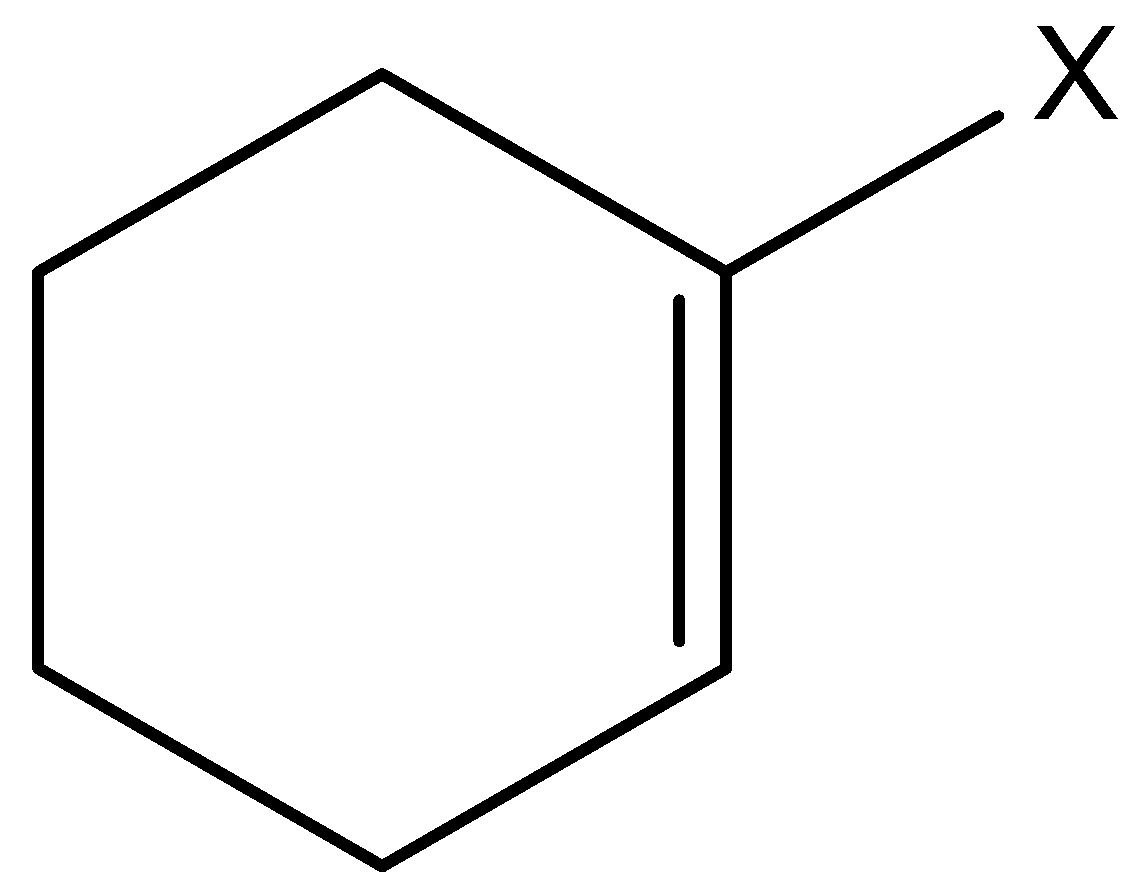
Let’s understand vinylic halide and aryl halides in detail. This is the type of halide in which the halogen atom is bonded to an sp2 hybridized carbon atom of a carbon-carbon double bond.
Aryl halides are the compounds in which a halogen atom is bonded to a sp2 hybridized carbon atom of the aromatic ring.
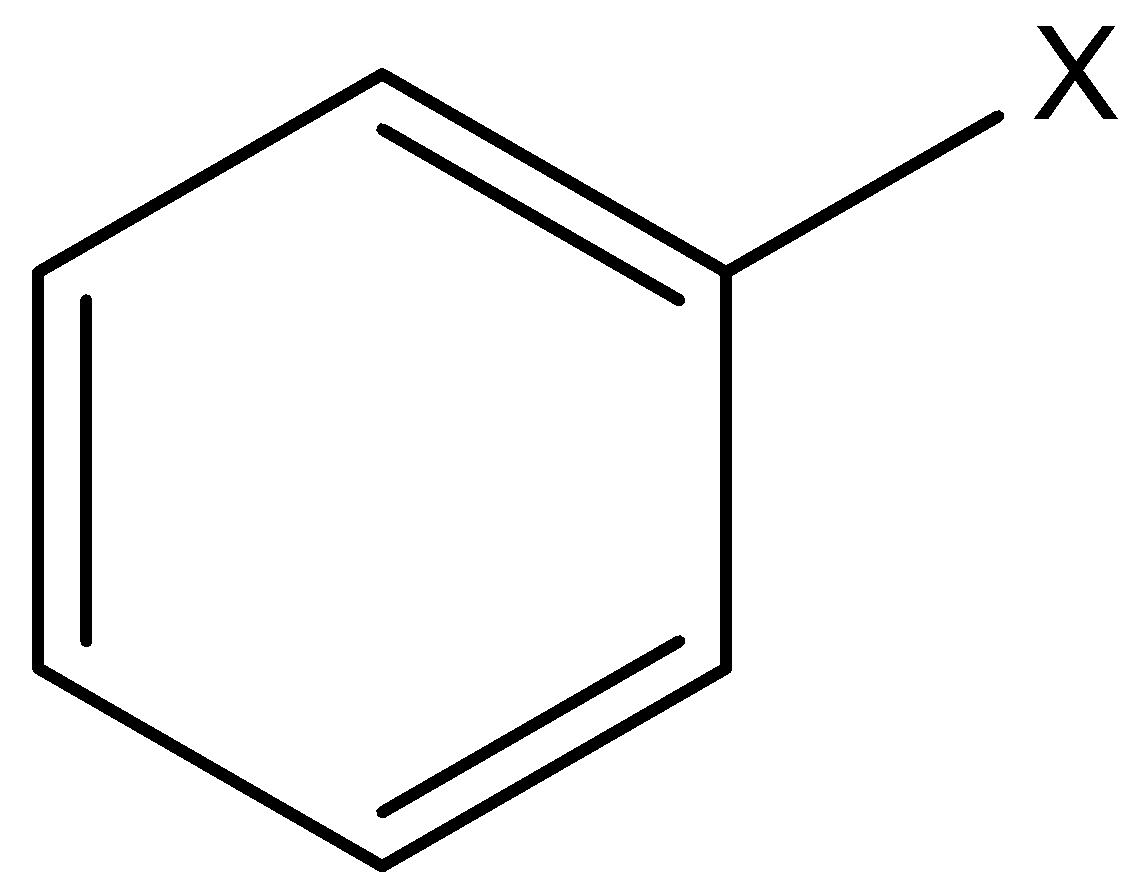
X is any halide (F, Cl, Br, I)
Now, come to the question. Here, aryl halide undergoes reaction with aqueous potassium hydroxide. The nucleophilic substitution reaction is difficult. Let’s consider chloro benzene.
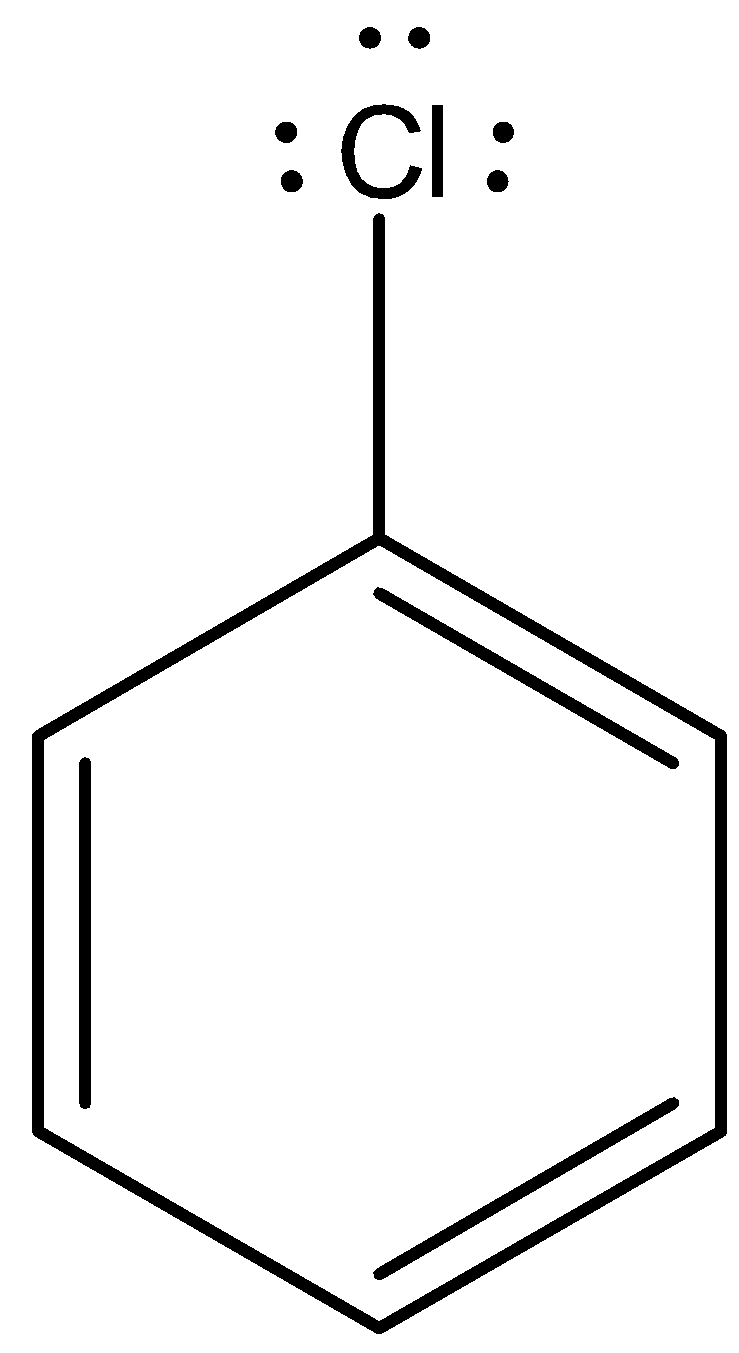
Chlorobenzene is an example of aryl halide. There are three lone pairs on the chlorine atom. The chlorine atom gives the lone pair to the neighboring carbon atom and the C-Cl bond acquires partial double bond character.
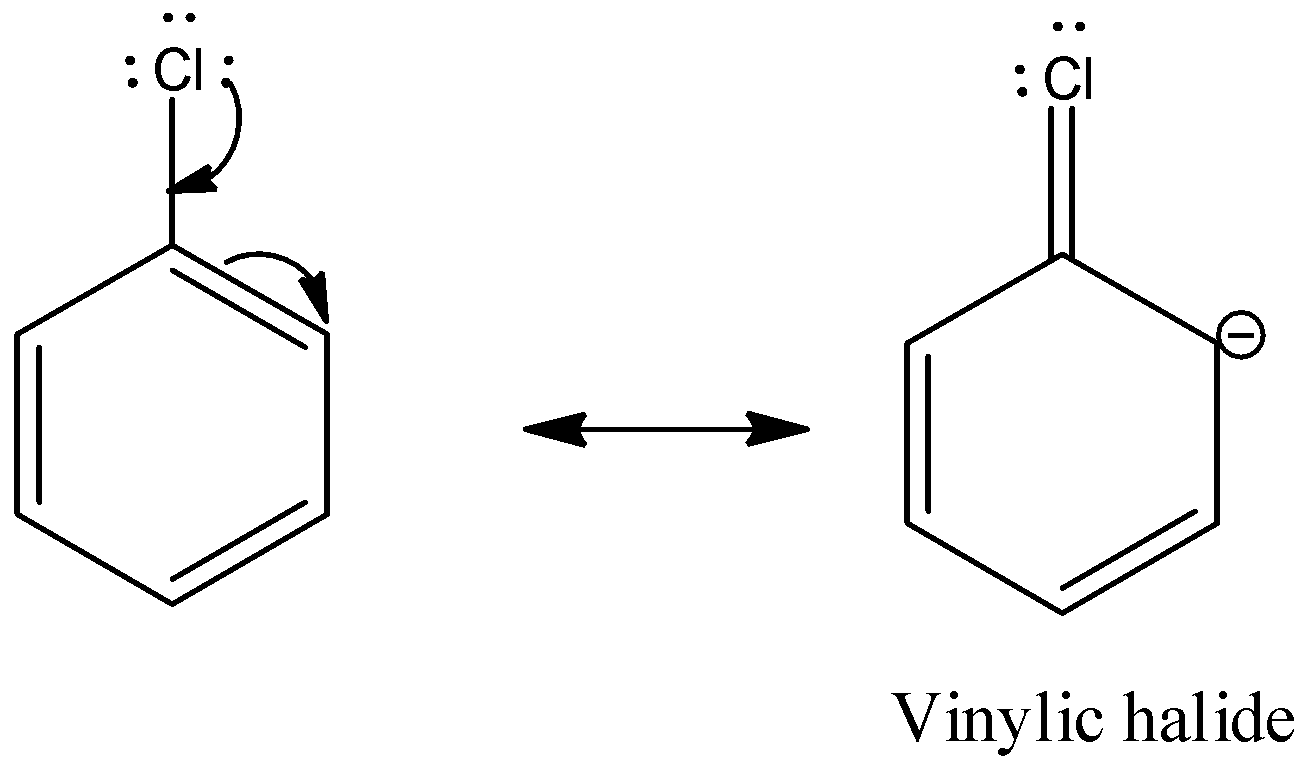
So, the substitution of nucleophiles is difficult.
So, we find that any aryl halides in nucleophilic substitution reaction resembles the vinylic halide.
Hence, the correct answer is option A.
Note:
On the basis of sp3C−X type of carbon halogen bond, alkyl halides are classified into three types alkyl halide, allylic halide and benzylic halides. Alkyl halides are those in which an alkyl group is bonded to a halogen group (ex methyl chloride). Allylic halides are those halides in which a halogen atom is bonded to an sp2 hybridized carbon atom next to carbon-carbon double bond.
Example: