Question
Question: In \(ICl_4^ - \) , the shape is square planar, the number of bond pair-lone pair repulsion at \({90^...
In ICl4− , the shape is square planar, the number of bond pair-lone pair repulsion at 90∘ are:
A. 6
B. 8
C. 12
D. 4
Solution
We can predict the number of bond pair-lone pair repulsions at90∘ by drawing its structure. The shape of ICl4− is square planar because it has four bond pairs and lone pairs. The geometry is octahedral.
Complete step-by-step answer: Before solving the question we have to look at the structure to determine their number of bond pair-lone pairs.
Now, the structure of ICl4− is,
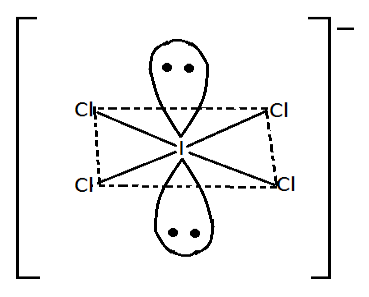
Iodine (I) has 7 electron it its valence shell in ICl4−eight electron participate in formation of compound
Shape of ICl4−is square planar. Since its geometry is octahedral, lone pairs reside above and below the square planar. In ICl4−each lone pair interacts with the 4 bond pair.
So, Number of bond pair-lone pair repulsion=Total number of lone pairs multiplied by number of bond pairs by which they interact. We have two lone pairs and four bond pairs
Number of bond pairs-lone pairs repulsion=2×4
=8
Thus, there is 8 bond pairs-lone pairs repulsion at 90∘ . So, the correct option is B.
Note: Valence Shell Electron Pair Repulsion Theory (VSEPR) .This is a very useful theory to predict the geometry or shape of a number of polyatomic molecules or ions on a non-transition element. This theory says that shapes of a species depend on the number of and nature of electron pairs surrounding the central atom of a species.
Table summarizes the relationship between the number of electron pairs that are bond pairs and lone pairs and the shape or geometry.
| Total number of electron pairs | Shape |
|---|---|
| 2 | Linear |
| 3 | Trigonal planar |
| 4 | Tetrahedral |
| 5 | Trigonal bipyramidal |
| 6 | Octahedral |
| 7 | Pentagonal bipyramidal |
