Question
Question: In \(ICl_4^ \ominus \) the shape is square planar. The number of bond pair-lone repulsion at \(90^\c...
In ICl4⊖ the shape is square planar. The number of bond pair-lone repulsion at 90∘ are:
(A). 6
(B). 8
(C). 12
(D). 4
Solution
We know that ICl4⊖ is AB4E2 type molecule and it shows:
Electronic Structure: AB4E2
Electronic Geometry: Octahedral
Hybridization of central atom is sp3d2
Complete step by step answer:
There are two lone pairs of electrons which are perpendicular to the square plane. In the square plane there are 8 lone pair electrons.
Thus, repulsion at 90∘=8
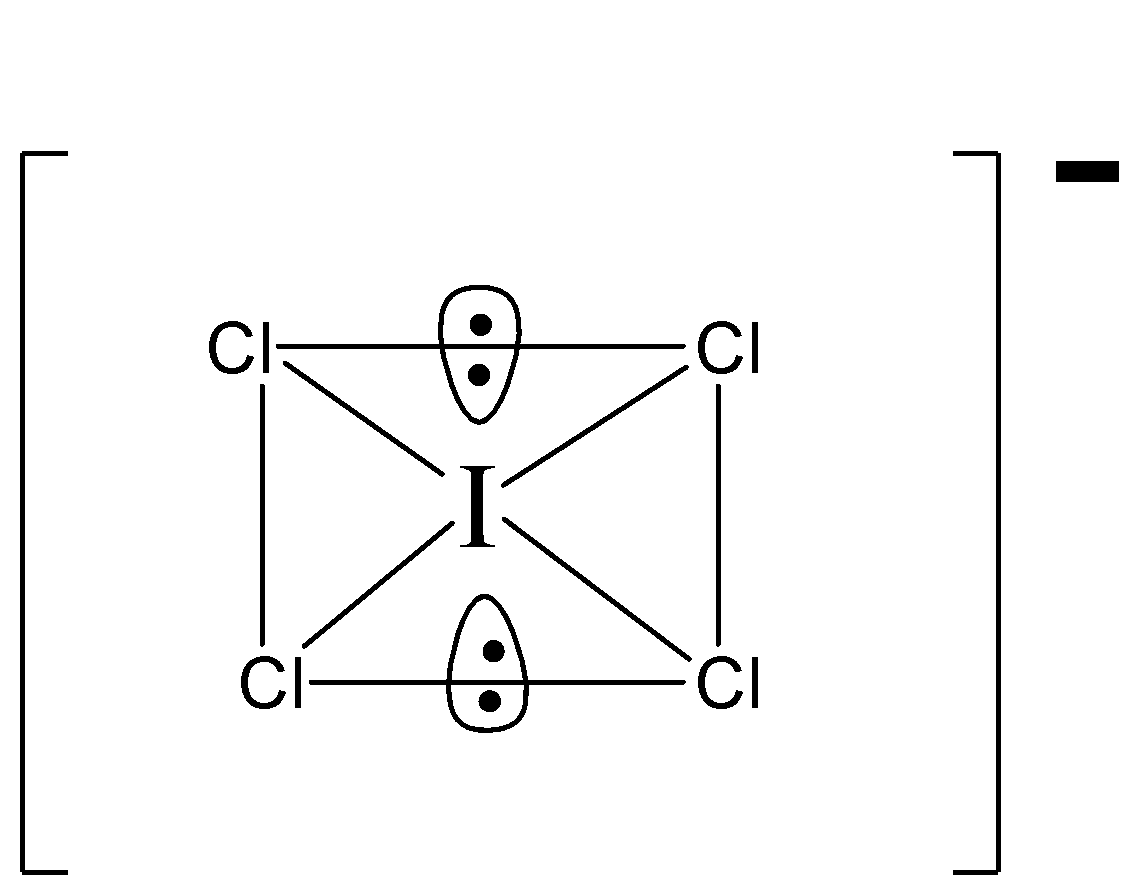
Therefore, the correct option is (B) 8.
Additional Information:
In the lewis structure of ICl4⊖ there are total 36 valence electrons.
Since Iodine (I) is below period 3 on the periodic table, it can have more than 8 electrons. In the lewis structure of ICl4⊖ the iodine atom has 12 valence electrons.
3−D structure.
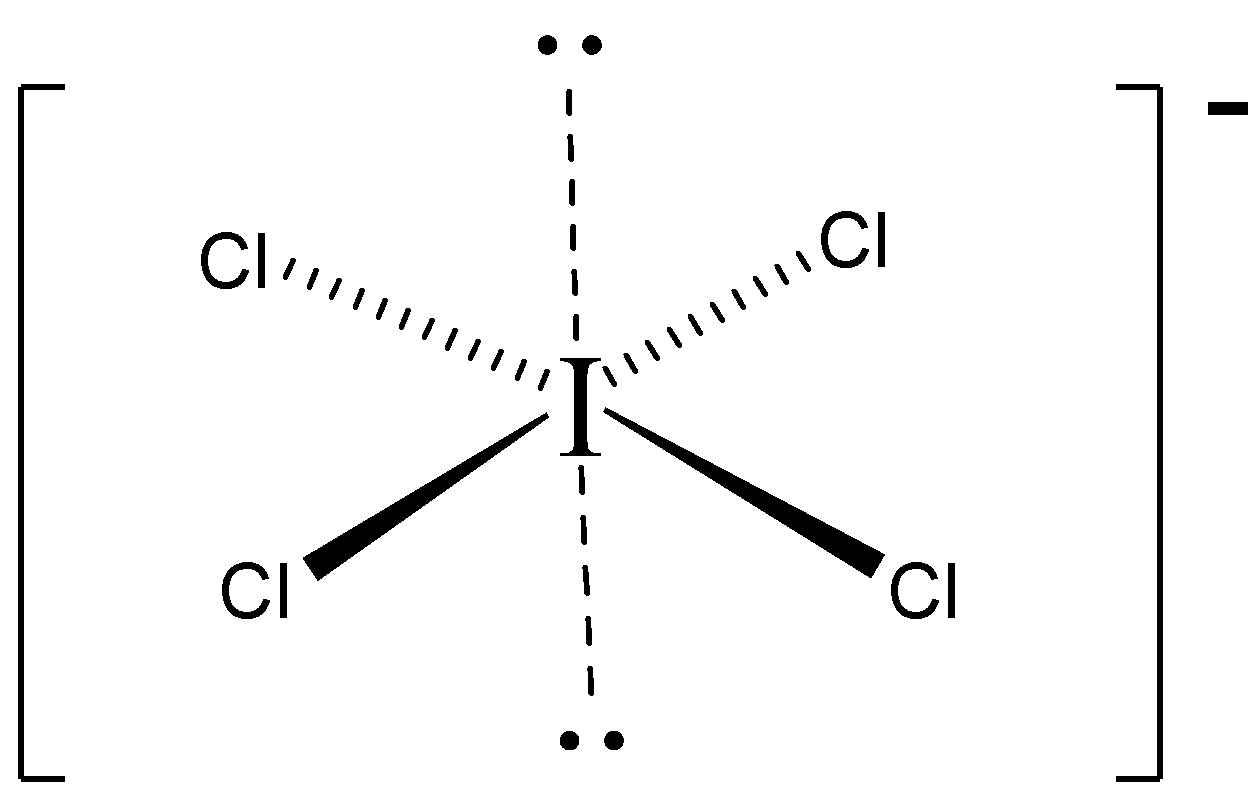
Important Points:
In ICl4⊖ lewis structure, Iodine (I) has the least electronegativity and goes in the center of lewis structure.
The ICl4⊖ lewis structure you’ll need to put more than eight electrons on the iodine atom.
In the lewis structure for ICl4⊖, there are a total of 36 electrons.
Note: Note that you should put ICl4⊖ lewis structure in brackets with −1 charge outside to show that it is an ion with negative one charge.
