Question
Question: In \(IC{I_4}^ - \) the shape is square planar. The number of bond pair-lone pair repulsion at \(90^\...
In ICI4− the shape is square planar. The number of bond pair-lone pair repulsion at 90∘ are:
A.6
B.8
C.12
D.4
Solution
The hybridisation of ICI4− is sp3d2 . It has 7 electrons in the outermost shell out of which 4 of them are used up to form 4 bonds with Cl.
Complete step by step answer:
ICI4− has the square planar shape with five nuclei. Its hybridisation is sp3d2 . It has Iodine as the central atom having 4 covalent bonds and two lone pairs. There is a single negative charge. The structure of ICI4− is:
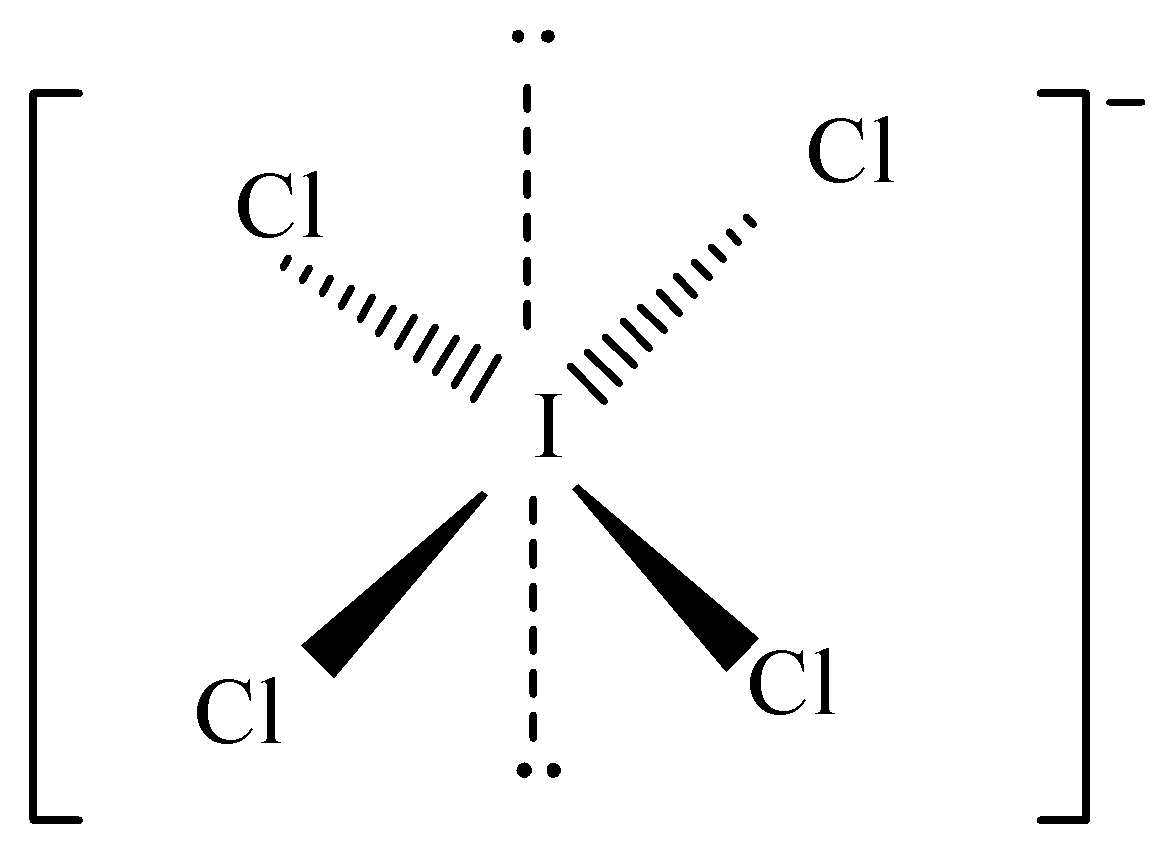
There are six electron groups around the I atom where four of them are bond pairs (BP) in the equatorial plane and two lone pairs (LP) of electrons in the axial positions and all are perpendicular to the square plane. Each lone pair is perpendicular to bond pairs.
So, the number of bond pairs – lone pair repulsion at 90∘ are 2×4=8 .
Therefore, the correct answer is option (b).
Note: ICI4− is a non-polar ion and it has an octahedral electron – pair geometry. Total axes of symmetry in ICI4− is 6.
