Question
Question: In how many of the following molecules, nitrogen can be estimated completely by using Kjeldhal metho...
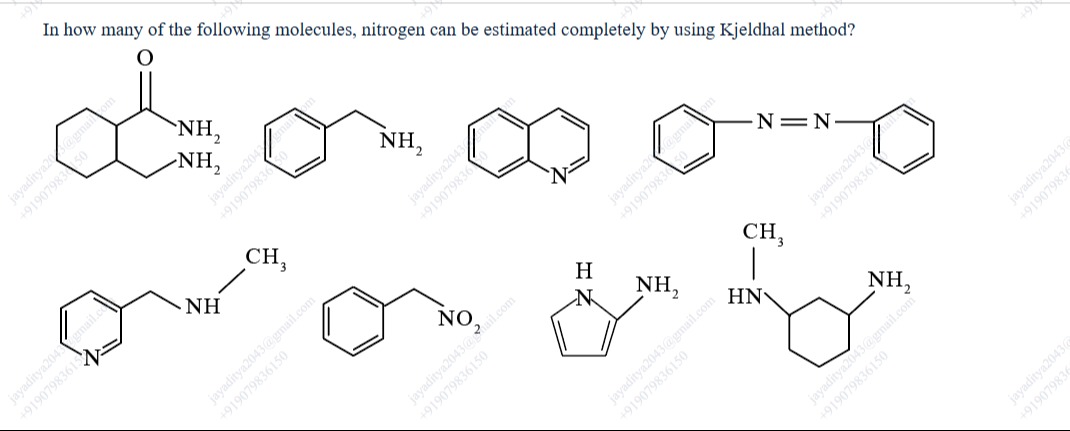
In how many of the following molecules, nitrogen can be estimated completely by using Kjeldhal method?
A
2
B
3
C
4
D
5
Answer
4 molecules
Explanation
Solution
Solution:
- Molecule 1: Contains two –NH₂ groups bonded to a carbonyl carbon (a urea‐like moiety). Under Kjeldahl digestion, amide–N (as in urea) is converted to NH₄⁺.
- Molecule 2: Benzylamine (–CH₂NH₂) is an aliphatic primary amine; it is fully converted to ammonium.
- Molecule 3: The nitrogen is part of a pyridine ring. Aromatic ring‐nitrogen (as in pyridine) is resistant to complete conversion under standard Kjeldahl conditions.
- Molecule 4: Contains a diazo (–N=N–) linkage; such nitrogen is not converted to ammonia.
- Molecule 5: Has two nitrogen atoms—a pyridine nitrogen (not completely digested) and an aliphatic methylamino group. Since one nitrogen remains unconverted, the total nitrogen is not estimated completely.
- Molecule 6: The –NO₂ (nitro group) does not yield NH₄⁺ in Kjeldahl digestion.
- Molecule 7: Although it has an –NH₂ group attached, its pyrrole ring nitrogen (due to aromatic stabilization) is not fully converted.
- Molecule 8: An aliphatic amine (N-cyclohexylmethylamine) is completely digested.
- Molecule 9: Cyclohexylmethylamine (–CH₂NH₂) is also completely digested.
Thus, only molecules 1, 2, 8, and 9 produce ammonia quantitatively on digestion.
Quick Core Explanation:
- Molecules with aliphatic amine or urea (amide) groups (1, 2, 8, 9) yield NH₄⁺ completely under Kjeldahl digestion.
- Heterocyclic, diazo, and nitro–nitrogen (in 3, 4, 5 [partially], 6, 7) are not completely converted.
