Question
Question: In haloarene compounds, halogen combines with carbons having which hybridisation? A. \(s{p^2}\) ...
In haloarene compounds, halogen combines with carbons having which hybridisation?
A. sp2
B. sp3
C. sp
D. dsp2
Solution
As we all know that haloarenes are the aromatic hydrocarbon compounds in which one or more than one hydrogen atom that is directly bonded to the aromatic ring are replaced by the halogen atoms. Hybridisation is basically the chemical bonding which is used to explain the covalent bonds in organic molecules.
Complete step by step answer:
The Haloarenes are the aromatic hydrocarbons in which hydrogen atoms are replaced by halogen atoms and these halogens are directly attached to aromatic hydrocarbons like Chlorobenzene.
sp hybridisation is the mixing of one s and one p orbital in the same shell and results in the formation of a new orbital called sp orbital.
sp2 hybridisation involves the mixing of one s and two p orbitals and the shape created by this mixing of orbitals is trigonal planar and the newly formed orbital is called sp2 hybrid orbital.
sp3 hybridisation is the mixing of one s and three p-orbitals resulting in the formation of four new equivalent orbitals giving the molecule a tetrahedral geometry.
dsp2 hybridisation is the mixing of one d-orbital, one s and two p-orbitals giving the molecule a square planar geometry and the orbital formed is called the dsp2 hybridised orbital.
For example- The carbon in chlorobenzene has three sigma-bonds and one pi-bond.
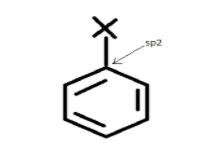
Generally, halogens combine with carbons in haloarenes which are sp2 hybridized.
**Therefore, the correct answer is (A) option.
Note: **
The simplest way to identify the hybridization in the given compounds is to count the number of sigma and pi bonds because these bonds are basically what makes the orbitals and the halogen atom when attaches to the carbon atom next to the aromatic ring it is generally sp3 hybridised.
