Question
Question: In \( {{H}_{2}}O, \) the bond angle \( HOH \) is \( {{104}^{\circ }}28\prime ~ \) but in \( {{H}_{2}...
In H2O, the bond angle HOH is 104∘28′ but in H2S, H2Se and H2Te the bond angles are pretty close to 90∘. This suggests that:
(A) oxygen uses sp2 hybrid orbitals to bond with the two hydrogen atoms while S,Se and Te use sp3 hybrid orbitals for bonding with the hydrogen atoms.
(B) oxygen uses sp3 hybrid orbitals to bond with the two hydrogen atoms while S,Se and Te use almost pure p orbitals
(C) oxygen uses sp3 hybrid orbitals to bond with the two hydrogen atoms while S,Se and Te utilize d orbitals for bonding with the hydrogen atoms.
(D) All the atoms use pure p orbitals to bond with the hydrogen atoms.
Solution
Hint : VSEPR (Valence shell electron repulsion theory) explains the minimal variations we can see from the theoretical bond angles in some of the molecules. As the name suggests it involves repulsion between valence shell orbitals.
Complete Step By Step Answer:
The traditional textbook explanation would argue that the orbitals in the water molecule is close to being sp3 hybridized, but due to lone pair - lone pair electron repulsions, the lone pair-lone pair angle opens up slightly in order to reduce these repulsions, thereby forcing the H−X−H angle to contract slightly. So instead of the H−O−H angle being the perfect tetrahedral angle 109.5∘ it is slightly reduced to 104.5∘ We know and we can see that oxygen has two lone pairs on itself. The water molecule has hybridization that means it is supposed to have bond angles of but there is a special case. In terms of repulsion, lone pair-lone pair repulsion is higher than any other repulsion. So, bond pair-bond pair repulsion and lone pair-bond pair repulsion is lower than lone pair- lone pair repulsion. So, due to lone pair-lone pair repulsion of two lone pairs of the oxygen atom, they both tend to repulse each other and try to stay as far as possible to each other. In that case, the bond angles of sp3 hybridization do not remain same and have less angle due to that because there is an increase in the angle between two lone pairs and hence there is a decrease in H−O−H angle. So, that is the reason why Water has H−O−H bond angle of 104.5∘ and not 109.5∘ .
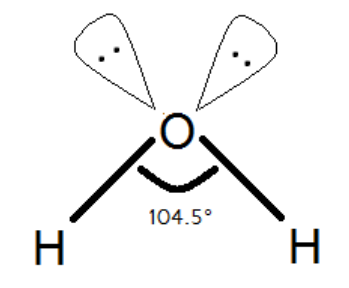
sp3 orbitals have an angle of 109∘28′ which hints that O is using this hybridisation to bond whereas the pure p orbitals are at 90 degrees with each other hinting that S,Se and Te use almost pure p orbitals. So in water, the orbitals in 2(O−H) bonds are roughly sp3 hybridized, but one lone pair resides in a nearly pure p−orbital and the other lone pair is in roughly sp3 hybridized orbital.
Therefore, correct answer is option B i.e. oxygen uses sp3 hybrid orbitals to bond with the two hydrogen atoms while S,Se and Te use almost pure p orbitals.
Note :
Remember that oxygen atoms have two lone pairs in the structure of water molecules. Do not forget to consider the lone pair while predicting the shape or bond angles of a molecule.
