Question
Question: In given reaction, product B is: 
A. But-2-ene
B. Butyne
C. But-2-yne
D. Butene
Solution
Generally alcoholic KOH acts as a strong base and removes the acidic hydrogen from the organic compounds easily. Alkyl halides undergo reaction with alcoholic KOH and form a dehydro halogenated compound as the product.
Complete step by step answer:
- In the question it is given what is the product B in the given reaction.
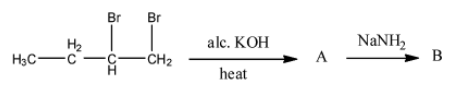
- In the given reaction there are two steps.
Step-1:
- We know that alc. KOH acts as base and removes the acidic hydrogens present in the given compounds.
- The chemical reaction of 1,2-dibromo butane with alc. KOH is as follows.
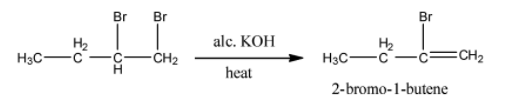
- The product formed in the above chemical reaction contains a double bond in between carbon-1 and carbon-2.
- The name of the compound formed in step-1 is 2-bromo-1-butene.
Step-2:
- The product formed in the step-1 reacts with sodamide and the chemical reaction is as follows.
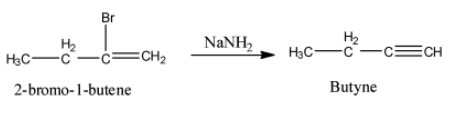
- In the above chemical reaction 2-bromo-1-butene reacts with sodamide and forms a product called butyne.
- The total given chemical reaction is as follows.
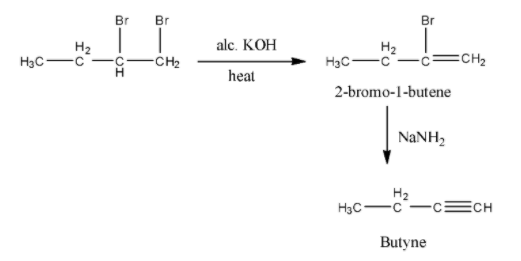
- There the product B formed in the given reaction is Butyne.
So, the correct option is B.
Note: Alcoholic KOH selectively removes the acidic hydrogens present in the given compound. Alc. KOH is a strong base when compared to normal potassium hydroxide (KOH). If the organic compound does not contain acidic hydrogens then there is no action of alc. KOH on it. In organic chemistry alc. KOH has a good role in synthesis.
