Question
Question: In \([Fe{(CN)_5}NO]\), sodium nitroprusside __________ (The question has multiple correct answers)...
In [Fe(CN)5NO], sodium nitroprusside __________
(The question has multiple correct answers)
A. Oxidation state of Fe is +2
B. This has NO+ as ligand
C. d2sp3- hybridization
D. None of the above is correct
Solution
We have to remember that the sodium nitroprusside is a chemical compound used for medical purposes. It is used to cure heart disease like heart failure to increase cardiac output. It is an inorganic compound formed by digesting a solution of potassium ferrocyanide in water with nitric acid.
Complete step by step answer:
We know that ligands are ions or molecules which are strongly bound to the central metal atom to form coordination complexes. The ligands present in the complex structure are responsible for the high reactivity of the compound as a whole.
Let us look at the chemical structure of the compound – sodium nitroprusside,
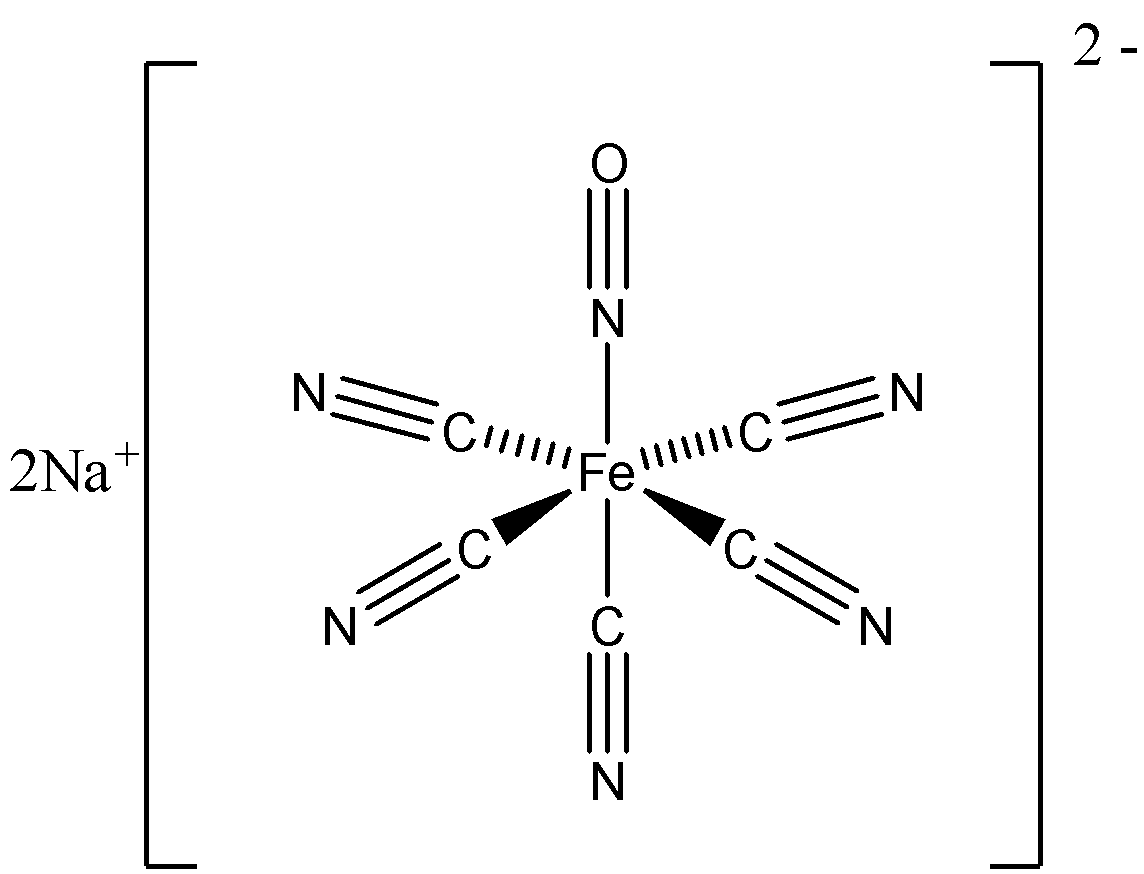
The sodium atom is bonded to a complex anion – nitroprusside. Nitroprusside contains one central iron atom bonded to five cyanide ligands and one nitric oxide ligand.
So here, the ligand present is cyanide and nitric oxide.
The structure of nitroprusside is octahedron.
Now let us look at the oxidation state of iron atoms. Iron atoms generally exist in either 2 or 3 numbers of oxidation. To calculate the number of oxidations let us assume the oxidation number of iron to be as ‘x’.
We know the oxidation number of other compounds, so altogether we have,
CN=−1
NO=+1
Fe=x
There are five cyanide ligands, one nitric oxide and one iron atom, and the oxidation of complete anion is -2.
Hence, we can write the expression as,
−2=5×CN+1×NO+Fe
Now we can substitute the oxidation states we get,
⇒−2=5×(−1)+1+x
−2=−5+1+x
⇒x=−2+5−1
On simplification we get,
⇒x=+2
Hence, the oxidation number of iron is +2.
We have to know that the anion compound exhibits an octahedron geometry since there are four linear planar and one above and one ligand below. The hybridization of an octahedron geometry is written as d2sp3.
So, the correct answer is Option A,C,B.
Note: We must remember that the sodium Nitroprusside is an organic and highly toxic compound. It is formed as a blood red colored salt which is used for curing heart related diseases and congestion problems. It helps to lower blood pressure. It can produce harmful effects if used in excess. Since the cyanide present in the compound is a poison and can slowly produce toxicity in the body. It is stored in a dark place so that the compound doesn’t degrade.
