Question
Question: In Dow’s process for manufacture of phenol, PhCl is fused with NaOH at elevated temperature under pr...
In Dow’s process for manufacture of phenol, PhCl is fused with NaOH at elevated temperature under pressure.
PhClNaOH623K,300atm[Intermediate]H2OPhenol(A)+(B+C)sideproduct
Which of the following statements are correct?
(This question has multiple correct options)
(A) Phenol is formed via the formation of benzyne intermediate
(B) p – phenyl phenol is also formed as a by product
(C) Diphenyl ether is also formed as a by – product
(D) Biphenylene is also formed as a by – product
Solution
Dow’s process is hydrolysis of chlorobenzene. It is a nucleophilic substitution reaction. It is formed by an intermediate of benzene. Nucleophilic substitution is a type of reaction in which an electron rich compound replaces a leaving group.
Complete answer:
- We refer to Dow's Process to the hydrolysis of chlorobenzene for the preparation of phenol.
-We can easily convert benzene chlorobenzene by electrophilic aromatic substitution.
- Dow’s process is preparation of phenol from haloarenes, it is an industrial preparation of phenol.
- Chlorobenzenes with 10% NaOH solution is heated at 623K under 300 atm pressure.
- The major product of Dow’s process is phenol but other by- products are also formed as a result of reaction between the species formed during the reaction.
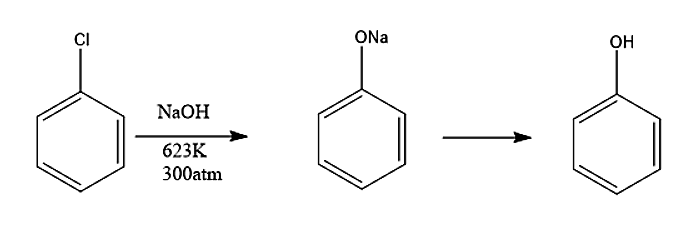
- Now let’s understand the reaction mechanism:
-In the first step, the base (NaOH) removes one hydrogen from the benzene ring and forms a carbanion which loses Cl− ion to form benzyne intermediate.
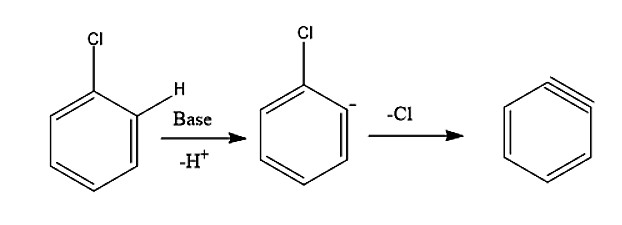
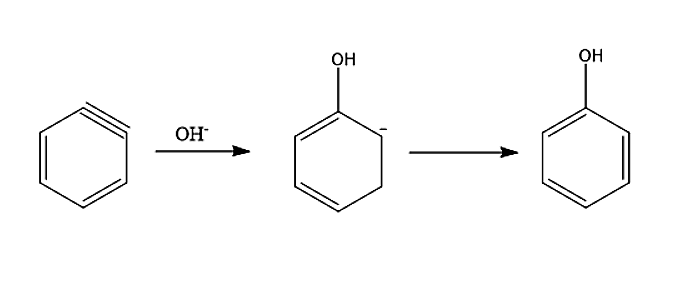
-In the second step, an attack of nucleophile takes place. The benzyne intermediate formed gets attacked by nucleophile OH−to form carbanion which subsequently gets protonated to form phenol.
- Now for side products, let’s see the conversion of reactants into reacting species.
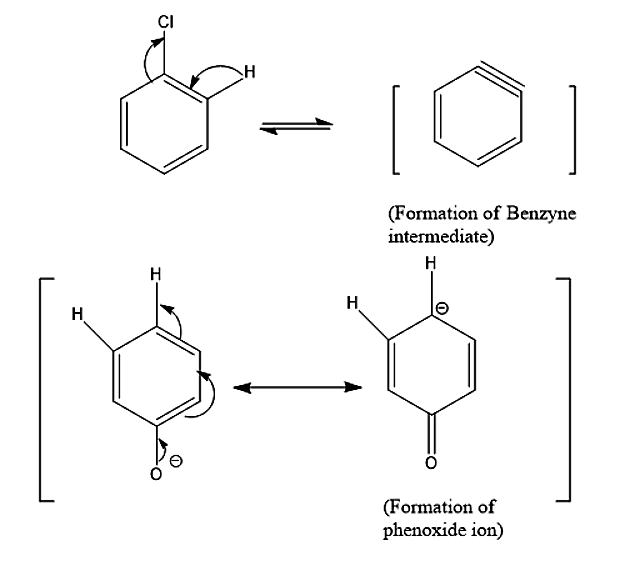
- Due to the presence of phenoxide ions diphenyl ether is also a by – product.
- Another by – product we see is para- phenyl phenol which is formed by benzyne and phenoxide ions.
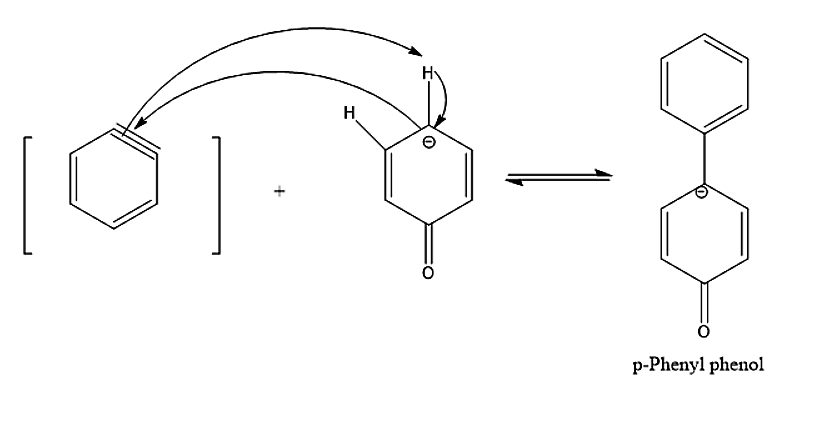
Therefore, the correct statements are options A. Phenol is formed via the formation of benzyne intermediate, B. p – phenyl phenol is also formed as a by-product, C. Diphenyl ether is also formed as a by – product.
Note:
-After the formation of benzyne, OH− nucleophilic attack takes place.
-The reaction mixture also contains some phenoxide ions. So, Diphenyl ether is a possible side product.
- The major product of Dow’s process is phenol but other by- products are also formed as a result of reaction between the species formed during the reaction.
