Question
Question: In diborane, the two H-B-H angles are nearly: \[ A.{60^0}C,{\text{ }}{120^0}C \\\ B.{90^0}...
In diborane, the two H-B-H angles are nearly:
A.600C, 1200C B.900C, 1200C C.950C, 1500C D.1200C, 1800CSolution
The structure of diborane is very simple; it has 2 boron atoms bonded to 6 hydrogen atoms. The two boron atoms are sp3 hybridized. There is also the presence of two bridges consisting of B-H-B. The bonds between the bridges are weak whereas between the four B-H are strong covalent bonds.
Complete answer:
The chemical formula of diborane is B2H6. It is a colorless, pyrophoric gas with a repulsively sweet odor. This compound is highly unstable at room temperature. The compounds consisting of boron and hydrogen atoms are called boranes. Diborane is one of the simplest boron hydrides.
Structure of diborane
The structure of the Diborane molecule consists of four hydrogen atoms and that of two boron atoms coming on the same plane. In between these planes, there are said to be two dividing atoms of hydrogen.
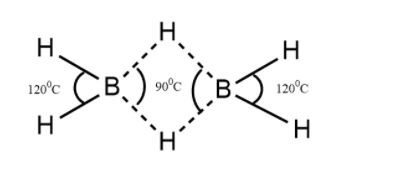
In the image, the two H-B-H angles are around 900C and 1200C
Therefore the correct option is B.900C, 1200C
Note:
Diborane is said to be a colorless and highly flammable type of gas at room temperatures. It mixes well with air and easily forms explosive mixtures. At high concentrations, it ignites rapidly in the presence of moist air at room temperature. It releases a huge amount of energy when burnt in the presence of oxygen. Diborane readily hydrolyzes in water to give hydrogen gas and boric acid.
