Question
Question: In diborane....
In diborane.
A
Four bridged hydrogen atoms and two terminal hydrogen atoms are present.
B
Two bridged hydrogen atoms and four terminal hydrogen atoms are present
C
Three bridged hydrogen atoms and three terminal hydrogen atoms are present
D
There are no bridged hydrogen atoms in diborane, only hydrogen bonds are present.
Answer
Two bridged hydrogen atoms and four terminal hydrogen atoms are present
Explanation
Solution
:
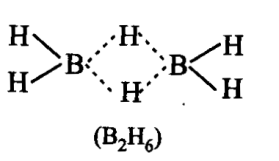
There are two terminal hydrogen atoms with one boron atom (total 4) and two hydrogen atoms are bridged between two boron atoms.
