Question
Question: In \(CO\) the hybridization state of \(C\) is:...
In CO the hybridization state of C is:
Solution
The orbital hybridization includes the concept of mixing of the atomic orbitals into new hybrid orbitals (with different energies, shapes, etc., than the individual atomic orbitals) which are suitable for the pairing of electrons to form chemical bonds in valence bond theory.
Complete step by step answer:
Hybrid orbitals are very useful in the explanation of geometry of the molecules and atomic bonding properties and are symmetrically oriented in space. Although sometimes taught together with the valence shell electron-pair repulsion (VSEPR) theory, valence bond and hybridization are in fact not related to the VSEPR model. Carbon and oxygen together have a total of 10 electrons in the valence shell. Following the octet rule for both carbon and oxygen, the two atoms form a triple bond, with six shared electrons in three bonding molecular orbitals, rather than the usual double bond found in organic carbonyl compounds. The electronic configuration of carbon is 1s22s22p2 and that of oxygen is 1s22s22p4 . This means that carbon has two p− orbitals to bond with oxygen and produce two sp hybridized orbitals. The carbon atom still has a vacant orbital and thus, receives a lone pair of electrons from the oxygen atom through back bonding and forms a coordinate bond. The carbon monoxide is a linear molecule with bond angle of 180o . The structure of carbon monoxide is as follows:
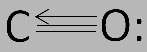
Thus, the hybridization of carbon atoms in CO is sp .
Note: Since four of the shared electrons come from the oxygen atom and only two from carbon, one bonding orbital is occupied by two electrons from oxygen, forming a dative or dipolar bond. This causes a C→O polarization of the molecule. The other two bonding orbitals are each occupied by one electron from carbon and one from oxygen, forming polar covalent bonds with a reverse C→O polarization, since oxygen is more electronegative than carbon.
