Question
Question: In \([Co{\left( {N{H_3}} \right)_6}]C{l_3}\) the number of covalent bond is: A.3 B.6 C.9 D.1...
In [Co(NH3)6]Cl3 the number of covalent bond is:
A.3
B.6
C.9
D.18
Solution
We can calculate the covalent bonds by multiplying the number of bonds formed within the ligand and the total number of ligand molecules.
Complete step by step answer:
As we know coordination complex as a compound that consists of a central atom (ion) that is metallic and is known as the coordination centre. It is enclosed by array bound molecules (or) ions called ligands (or) complexing agents. Transition metals are coordination complexes. A coordination complex whose centre comprises a metal atom is called a metal complex of d block element.
We can draw the structure of [Co(NH3)6]Cl3 as,
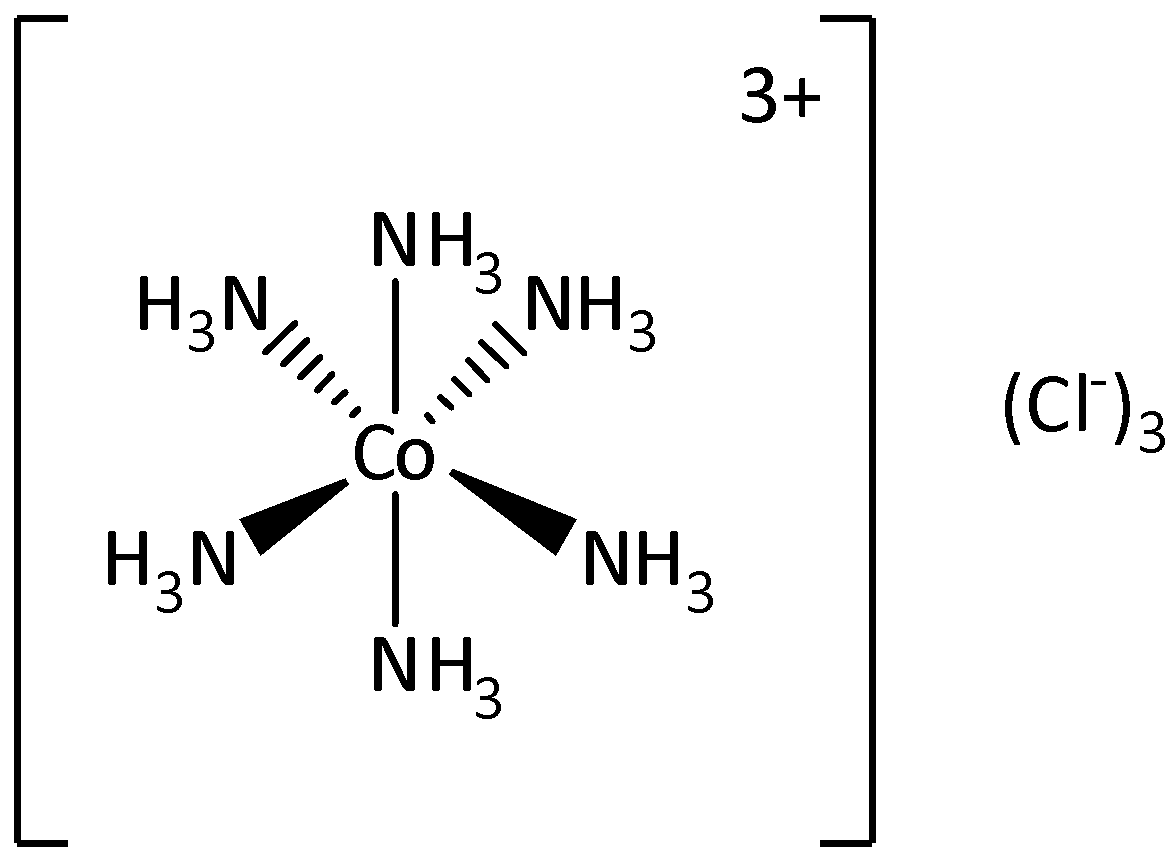
In the complex [Co(NH3)6]Cl3 we can see that the central metal atom is cobalt and the ligands are NH3 and chloride ions.
The covalent bonds could be calculated by multiplying the number of bonds formed within the ligand and the total number of ligand molecules.
The six molecules of NH3 are linked to cobalt through six coordinate covalent bonds. Each NH3 molecule is bonded to three atoms of hydrogen through covalent bonds.
The total number of ligand molecules is six and the number of bonds formed within NH3 is three. Each nitrogen atom is linked with each hydrogen atom by three covalent bonds.
The total number of N−H bonds is calculated as 6×3=18.
The number of covalent bonds in [Co(NH3)6]Cl3 is six.
So, the correct answer is Option D .
Note:
We must remember that the coordination compounds contain specific colours. Therefore, they are used in industries for intense colourations. Phthalocyanine is a class of coordination complexes that the dyes and pigments industry use.
EDTA is another complex compound that is used for determining the hardness of water. Coordination compounds could also be used as catalysts. These days, they are used in the polymer industry. Coordination compounds can be used in the extraction of metals from their ores. Extraction of nickel and cobalt by hydro-metallurgical processes requires a lot of complex ions.
