Question
Question: In \[Ca{{F}_{2}}\] type (fluorite structure), \[C{{a}^{2+}}\] ions from_(A)_ structure and F ions ar...
In CaF2 type (fluorite structure), Ca2+ ions from_(A)_ structure and F ions are present in all_(B)_voids. The coordination number of Ca2+ is (C) and F- is (D).
(A). (B), (C) and (D) respectively are:
A. (A)- ccp; (B)- octahedral; (C)-8; (D)-4
B. (A)- bcc; (B)- tetrahedral; (C)-4; (D)-8
C. (A)- ccp; (B)- tetrahedral; (C)-8; (D)-4
D. (A)- ccp; (B)- octahedral; (C)-4; (D)-8
Solution
Coordination number is also called Ligancy. The number of atoms, or ions that a central metal atom or ion holds as its nearest neighbors in a complex or in a coordination compound or in a crystal is called coordination number. There are two types of lattice structures, ccp and bcc.
ccp - cubic closest packing
bcc - body centered cubic lattice
Complete step by step answer:
In calcium fluoride (CaF2), the calcium ions (Ca2+) form the cubic closest packing (ccp) structure (let us assume as A) arrangement. The fluoride occupies all the corner positions of the cube and the center position of each face of the cube.
There are two tetrahedral positions present for each calcium ion and the fluoride ions (F−) occupies all the tetrahedral sites (let us assume as B). The stoichiometry of the calcium fluoride (CaF2) is 1:2.
Every fluoride in the cube is surrounded by four calcium ions (let us assume as D). While every calcium ion is enclosed by eight fluoride atoms (C) those are located towards the corners of the cube. Then the coordination of the calcium difluoride compound is 8:4.
The structure of CaF2 is as follows.
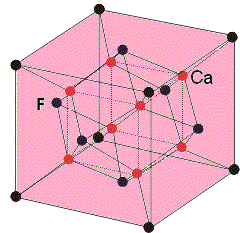
So, the correct answer is (A)- ccp; (B)- tetrahedral; (C)-8; (D)-4
So, the correct answer is “Option C”.
Note: Don’t be confused with calcium fluoride (CaF2), and fluorite. Both are the same. Fluorite (also called fluorspar) is the mineral form of calcium fluoride,CaF2. Calcium fluorite absorbs and neutralizes negative energy and stress.
