Question
Question: In \(C{r_2}O_7^{2 - }\) every Cr is linked to: A.two O atoms B.three O atoms C.four O atoms ...
In Cr2O72− every Cr is linked to:
A.two O atoms
B.three O atoms
C.four O atoms
D.five 0 atoms
Solution
Dichromate salts contain Cr2O72− dichromate anion. These are oxoanions of chromium with +6 oxidation state and they are good oxidizing agents as they are linked to oxygen atoms. Two chromium atoms are bonded with a single oxygen atom between them and the rest of the oxygens are bonded to individual chromium atoms in equal ratio.
Complete step by step answer:
Dichromate ion is a divalent inorganic anion .It is a chromium oxoanion and has a molecular formula Cr2O72− . It is a conjugate base of hydrogen dichromate. Dichromate ion is obtained by removing both protons from dichromic acid (H2Cr2O7) . It is used as an oxidizing agent. Due to its ionic property it forms many salts which can be used in many oxidation-reduction reactions.
The charge on the dichromate ion is -2 and the oxidation state of chromium in Cr2O72− is +6. The Cr6+ ion has 4s and 3d vacant orbitals . These orbitals combine to give sd3 hybrid orbitals. Thus, each Cr6+ ion in the dichromate (Cr2O72−) ion is sd3 hybridised. Due to this dichromate ion consists of two tetrahedrons sharing an oxygen atom at the common vertex. Therefore, each of the two chromium atoms at the centre of the tetrahedra bond to four oxygen atoms.
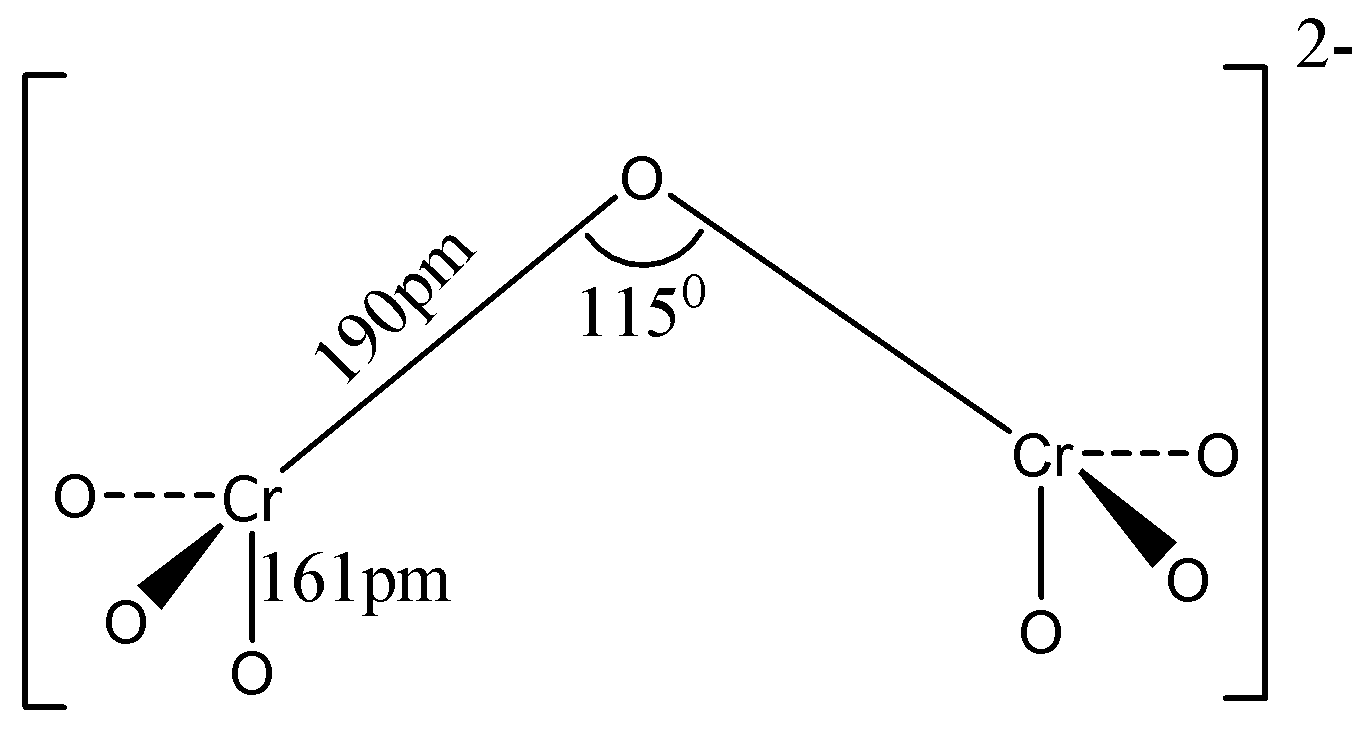
Hence, option (C) is the correct answer
Note: There are six Cr−O terminal bonds in dichromate ions. These bonds are equivalent due to resonance. The two Cr−O bonds which share an oxygen atom are longer than the other six equivalent Cr−O bonds. This defines the structure of dichromate anion along with bond angles.
