Question
Question: In \({{C}}{{{O}}_{{2}}}\) molecule, \({{CO}}\) bond is polar but \({{C}}{{{O}}_{{2}}}\) molecule is ...
In CO2 molecule, CO bond is polar but CO2 molecule is nonpolar because the vector sum of dipole moment of the two CO bond is zero.
A.True
B.False
Solution
The polarity of the bond is measured by its dipole moment. The molecule which has a dipole moment is called a polar molecule. The molecule which does not possess a dipole moment is called a nonpolar molecule. Molecules that have a polar covalent bond can be a polar or nonpolar molecule.
Complete step by step answer:
The dipole moment gives the measure of the polarity of the bond.
The dipole moment is a vector quantity.
It possesses both magnitude and direction.
Even if the molecule has polar bonds, if the dipole moment of the molecule is zero then the molecule is said to be non-polar.
If the molecule has a non-zero dipole moment then it is said to be a polar molecule.
When we consider the dipole moment of a molecule, we have to consider the vector sum of all dipoles in the molecule.
Whenever there is a separation of charge between two atoms in a bond, we can say that there exists a dipole.
Here in the question, it is said that the CO bond in the CO2 molecule is polar. We know that oxygen in the CO bond is more electronegative than carbon. So there is a separation of charge between carbon and oxygen in the CO bond.
Thus there exists a dipole in the CO bond of the CO2 molecule.
Since there exists a dipole in the CO bond of the CO2, we can say that CO bond is polar in the CO2 molecule.
As mentioned earlier dipole moment of the molecule is the vector sum of the individual dipoles in the molecule. That is the dipole moment depends both on magnitude and direction.
Therefore, for a molecule to be polar the vectors representing the dipole moment of each bond should not cancel out each other.
Here in the CO2 molecule, there is no net dipole moment since the dipole moment vectors of the two CO bond are in the opposite direction as shown below, it cancels out each other.
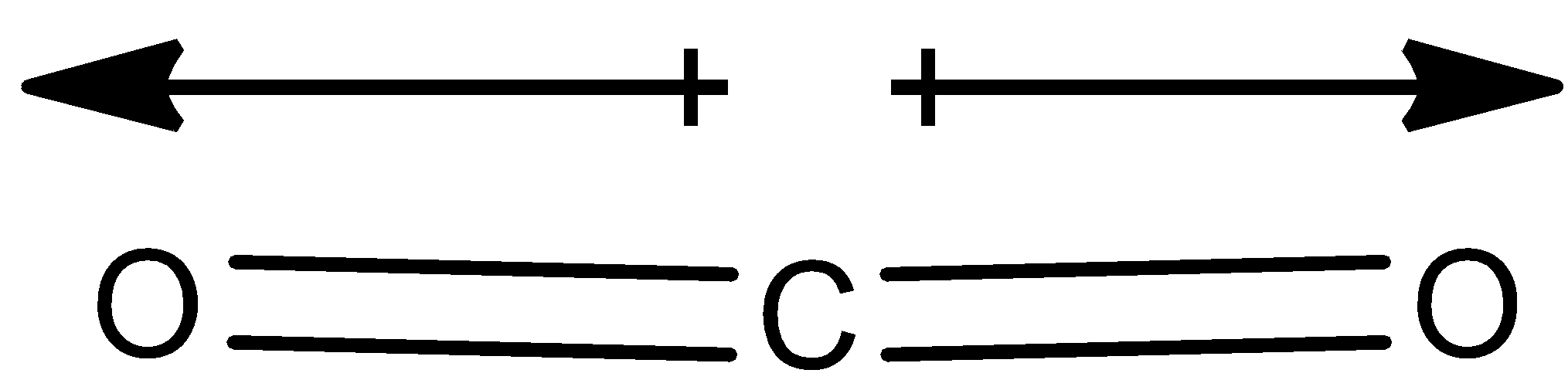
Therefore the net dipole moment of the CO2 molecule is zero.
Thus CO2 is non-polar.
The CO bond in the CO2 molecule is polar but the molecule CO2 is nonpolar because the vector sum of the dipole moment of the two CO bonds is zero.
Hence, the correct option is (A).
Note:
CO2 is a linear molecule. It is symmetric. Therefore the dipole moment of the two bonds cancel out each other and thus the net dipole moment is zero. Non-polar compounds are symmetric and polar molecules are asymmetric. Although the molecule contains a polar covalent bond, it is not a necessary condition for the molecule to be polar.
