Question
Question: In \(C{H_3}COOH\) molecule, the \(C - C\) bond is formed by: A. \(s{p^3} - s{p^3}\)overlap B. \(...
In CH3COOH molecule, the C−C bond is formed by:
A. sp3−sp3overlap
B. sp2−sp2 overlap
C. sp3−sp2 overlap
D. sp3−sp overlap
Solution
Acetic acid also known as ethanoic acid is an organic compound with chemical formula CH3COOH. The functional group present in this compound is carboxylic acid i.e. −COOH.
Complete answer
In acetic acid, we have two carbon atoms,4 hydrogen atoms and two oxygen atoms. Hybridisation is known as intermixing of atomic orbitals. These atomic orbitals must have the same energy and after the recombination their orbitals will lead to the making of hybrid orbitals. These hybrid orbitals are having equivalent energy. The total number of hybrid orbitals must be equal to the sum of all intermixing orbitals.
In sp hybridisation, one s orbital and one p orbitals will mix to form another two hybrid orbitals which are equivalent in same size and energy. This sp hybridisation is diagonal in shape. And it contains a 50% s and 50% p orbitals. In sp2hybridisation, one s orbital and two porbitals will mix to form three hybrid orbitals which are equivalent in same size and energy. This sp2 hybridisation is trigonal planar in shape. In sp3 hybridisation one s orbital and three p orbitals will mix to form another four hybrid orbitals which is equivalent in size and energy. This sp3 hybridisation is tetrahedral in shape.
In case of ethanoic acid, the carbon of methyl group combines with three hydrogen atoms and one adjacent carbon. In case of carbon 2p is the valence subshell and hydrogen has 1s valence subshell. So, there will be four s−p hybrid orbitals. So, the hybridisation of methyl carbon will be sp3.While in case of carboxylate carbon atom carbon forms three s−p hybrid orbitals. So, the hybridisation of carbon in the carboxylate group will be sp2. The structure of CH3COOH showing these hybridisations is shown below.
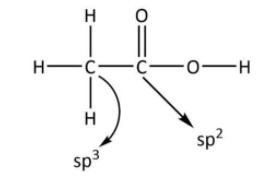
Hence, the C−C bond is formed by sp3−sp2overlap.
**Hence, option C is correct.
Note**
The examples of molecules with sphybridisation will be carbon dioxide, Beryllium chloride etc. And the examples of molecules with sp2hybridisation is boron trifluoride, ethylene. And the examples of molecules with sp3hybridisation will be methane, ethane etc.
