Question
Question: In \(C{{H}_{3}}C{{H}_{2}}OH\) , the bond that undergoes heterolytic cleavage most readily is: [A] ...
In CH3CH2OH , the bond that undergoes heterolytic cleavage most readily is:
[A] C – C
[B] C – H
[C] C – O
[D] O – H
Solution
To solve this question, firstly find out which of the following bonds will be the most ionic. You can compare the electronegativity to find out the ionic bond. Breaking of covalent bonds is difficult compared to ionic bonds. So, ionic bonds will undergo better heterolytic cleavage. Use this to answer the given question.
Complete step by step solution: We should know the compound given to us ethanol. Ethanol is a chemical compound with the chemical formula C2H5OH we can also write is as CH3CH2OH. As we can see, ethanol contains a –OH functional group, therefore it is termed as alcohol. Now, let us discuss what heterolytic cleavage is. The energy required for the breaking of a bond is known as the bond dissociation energy. It gives us the strength of a chemical bond. Stronger the bond, higher is the energy required to break the bond and thus higher is the bond dissociation energy.
Bonds can be broken either homolytically or heterolytically. Bond dissociation energy usually refers to the homolytic case. However, in a heterolytic bond cleavage, the bond pairs (the electron pairs that were used for the formation of the bond) are shared unevenly among the products. Now, let us draw the structure of ethanol and try to figure out which heterolytic cleavage can be readily formed.
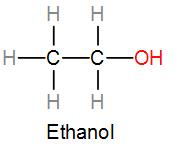
We know that C – C bonds are strong covalent bonds. So, are the C – H bonds. However, we can see that the electronegativity difference between oxygen and the hydrogen atom is the highest. So, the O – H bond is the most ionic. We know that ionic bonds break more easily than covalent bonds. So, here the O – H will break more readily.
Therefore, the correct answer is option (D) O – H
Note: In the above discussion, we have discussed heterolytic bonds. However, in a homolytic bond cleavage, the two electrons which were engaged in the formation of the bond go back to their original atoms as the bond breaks. Ethanol is a simple alcohol and we can naturally produce it by fermentation of sugar by yeast but also synthetically through petrochemical processes. We commonly use ethanol as a chemical solvent to dissolve compounds which are insoluble in non-polar solvents.
