Question
Question: In \({{{C}}_{{3}}}^{{{4 - }}}\), the number of \({{\sigma }}\)- and \({{\pi }}\)- bonds present betw...
In C34−, the number of σ- and π- bonds present between carbon atoms are:
A.1σand1π
B.2σand2π
C.2σand1π
D.1σ bond only
Solution
Sigma bond is formed by the head-on overlap of atomic orbitals. It is the strongest covalent bond. It is formed by the overlap of orbitals in an end to end fashion. There are different types of sigma bonds which are s−s , s−p and p−p . This is an example of a p-p sigma bond. When 2 lobes of an atomic orbital overlap on the orbital of another atom, it is a bond or πbond. The Sigma bond is shown by the symbol σ and the bond by the symbol π .
Complete step by step answer:
We know that carbon has 4 valence electrons.
In this question, there are 3 carbon atom
So the parent chain can be drawn as C−C−C
The charge on the C3 is -4.
Let us take the middle carbon. We know that carbon has a valency of 4, thus it is attached to 2 carbon atoms with 4 bonds.
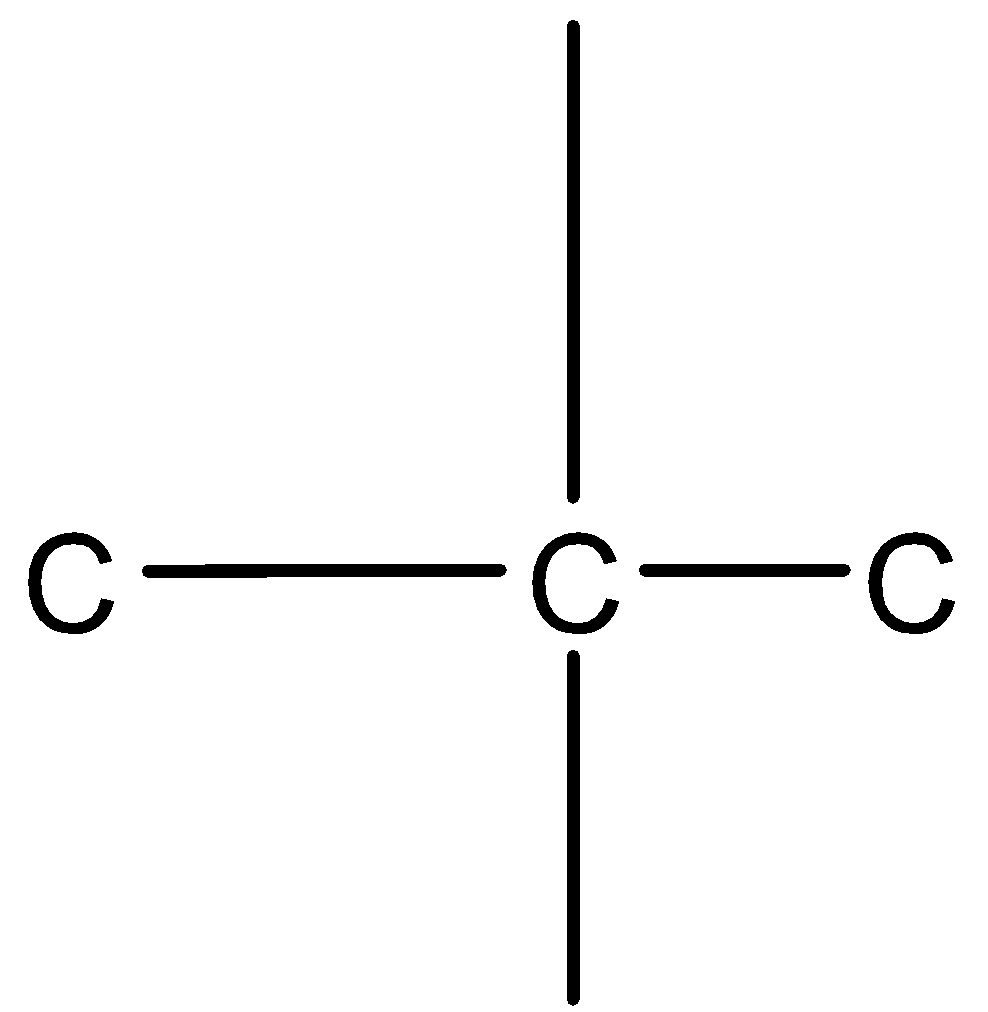
Since the middle carbon is attached to only 2 carbon atoms, its 2 valencies are shared with the adjacent two carbon atoms.
Now, there is no hydrogen atom and the molecule has a negative charge.
So, the remaining 2 lone valence electrons undergo lateral overlap forming 2 πbonds.
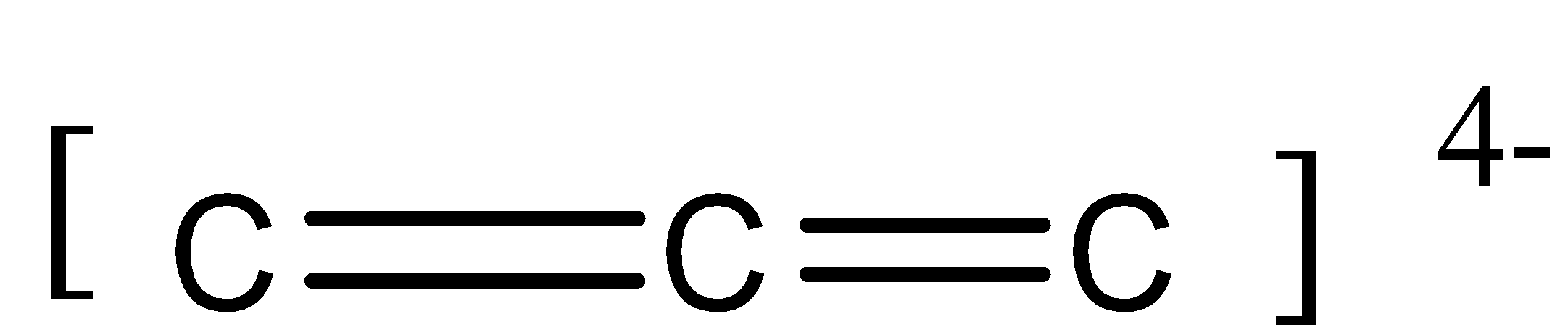
There is an overall −4 charge. It is due to the non-bonded 2 electrons on the first and third carbon atom.
Thus we can say that in this molecule, there is 2 sigma bond and 2 pi bond (2σbond and 2πbond).
The correct answer is an option (B).
Note:
When an example of hydrocarbon is taken, carbon with all σ bonds is sp3 hybridized. The carbon with 1 σbond and 1 π bond forms a sp2 hybridized and if the carbon is attached to 2 π bonds, then it is sp hybridized.
