Question
Question: In buna-S, the symbol ‘Bu’ stands for: (A) 1 – butene (B) n – butene (C) 2 – butene (D) Buta...
In buna-S, the symbol ‘Bu’ stands for:
(A) 1 – butene
(B) n – butene
(C) 2 – butene
(D) Butadiene
Solution
Buna – S is a random copolymer which is formed by the emulsion polymerization of a mixture of butadiene and styrene in the ratio of 1:3. This reaction takes place in the presence of a peroxide catalyst at 5∘ C and therefore the product is called cold rubber. The rubber obtained is also called Styrene butadiene rubber (SBR) or commonly known as Buna – S.
Complete Step-by-Step Solution:
Buna – S is a more commonly used name for a polymer named styrene butadiene rubber. The structure of Buna – S is given by:
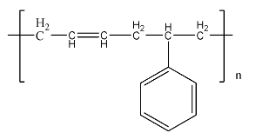
Buna – S is formed using the monomers butadiene and styrene. The reaction of this process can be given as:
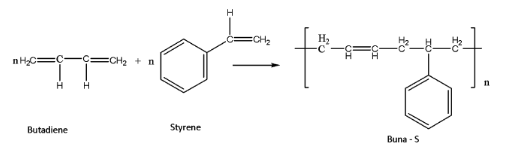
In the word Buna – S, the Bu stands for Butadiene, since butadiene is one of the constituent monomers of the given polymer. Also, Na stands for sodium and S represents the monomer Styrene used for the synthesis of the given polymer.
Hence, Option D is the correct option.
Note: Buna -S can be derived from the monomers, butadiene and styrene using two different processes. These processes involve polymer formation from either a solution or an emulsion. The process solution is known as S-SBR while the process which prefers emulsion is known as E-SBR.
