Question
Question: In \( BrF_3 \) , expected geometry is trigonal bipyramidal and actual geometry is T-shaped. True or ...
In BrF3 , expected geometry is trigonal bipyramidal and actual geometry is T-shaped. True or False.
Solution
Hint : Hybridization is the idea that atomic orbitals fuse to form newly hybridized orbitals, which in turn, influences molecular geometry and bonding properties. Hybridization is also an expansion of the valence bond theory.
Complete Step By Step Answer:
To determine the hybridization of bromine trifluoride we will first take the bromine atom which is the central atom and look at its electron configuration. It is represented as;
1s22s22p63s23p63d104s24p5
However, in order to form bonds with the fluorine atom some electrons in Bromine are shifted to 4d-orbitals. Besides, fluorine has a higher oxidative capacity and therefore it forces bromine to promote electrons to the said level. Now, bromine can use the d-orbitals for hybridization.
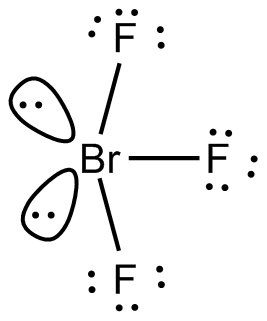
BrF3 Will consist of seven electrons in its outermost shell. After the bond formation, it will further have two lone pairs and 3 Br−−F covalent bonds. As the hybridization value or the electron pair is equal to five it gives rise to sp3d hybrid orbitals.
BrF3 Is AX5 type of molecule with two lone pairs and three bond pairs of electrons. The central Br atom undergoes sp3d hybridization. The expected geometry is trigonal bipyramidal and the actual geometry is T-shaped since two of the orbitals are occupied by lone pairs.
Note :
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles, and any other geometrical parameters that determine the position of each atom.
