Question
Question: In bisulphate ion, the formal charge on the sulfur atom is: A. \[+1\] B. \[+2\] C. \[+4\] D....
In bisulphate ion, the formal charge on the sulfur atom is:
A. +1
B. +2
C. +4
D. +6
Solution
Formal charge of an atom in a molecule can be found by the relation: formalcharge=valence electrons−(non−bonding valance electrons)−2(bonding electrons)
Using the above relation, find the formal charge on the sulfur atom in bisulphate ion.
Complete step by step answer:
We know that the relation to find the formal charge mathematical can be expressed as shown below:
formalcharge=valence electrons−(non−bonding valance electrons)−2(bonding electrons)
The structure of the bisulphate ion is as follows:
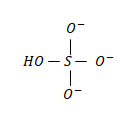
In which the middle atom is the sulfur atom.
The valence electrons of the sulfur atom is = 06 electrons;
The non-bonding valence electrons of the sulfur atom in the above molecule is = 0 electrons;
The bonding valence electrons of the sulfur atom in the above molecule is = 08 electrons.
Put all these values in the above formula and calculate the formal charge
After substituting all the values in the above relation we obtain
formalcharge=6−(0)−28 = +2
Therefore the required formal charge on the sulfur atom in the bisulphate molecule is +2
Hence option (B) is the correct answer.
Note: Formal charge can be defined as the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of the relative electronegativity.
The formal charge on an atom in a molecule reflects the electron count associated with the atom compared to the isolated neutral atom.
