Question
Question: In benzyne, the triple bond consists of: A. One \(sp-sp\) \(\sigma \)- bond and two \(p-p\) \(\pi ...
In benzyne, the triple bond consists of:
A. One sp−sp σ- bond and two p−p π- bonds
B. Two sp−sp σ- bonds and one p−p π- bond
C. One sp2−sp2 σ- bond and one p−p π- bond
D. One sp2−sp2 σ- bond, one sp2−sp2 π- bond, and one p−p π- bond.
Solution
Try to recall that in benzyne all the carbon atoms are sp2 hybridized and one π- bond is formed by hybridized orbitals. Now, by using this you can easily find the correct option from the given ones.
Complete step by step answer:
- It is known to you that benzyne is a neutral, highly intermediate in which aromatic characters are not markedly distributed.
- The benzyne contains a triple bond which is not like the triple bond of acetylene where the two carbon atoms form a σ- bond using sp orbitals and the remaining are used to form p−p π- bonds.
- Such a structure like acetylene triple bond is not possible in benzyne because of the hexagonal geometry associated with benzene ring. Most probably the new mount of benzene is formed by the sideways overlapping of sp2orbitals of the two adjacent carbon atoms. So, the triple bond contains one sp2−sp2 σ- bond, one sp2−sp2 π- bond and one p−p π- bond.
- This new bond orbitals lie along the side of the ring, and has little interaction with the pi cloud lying above and below the ring. Since the sideways overlapping is not very good, the new bond is weaker one and hence benzyne is highly reactive.
- The structure of benzyne is given below:
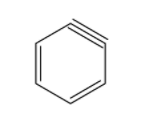
Therefore, from above we can conclude that option D is the correct option to the given question.
Note: Note that benzyne is formed from benzene via a free radical elimination reaction in which the pi-bond between the two carbon atoms is formed by sp2 hybridized orbitals. This is the only case in which a pi-bond is formed by hybridized orbitals.
