Question
Question: In Arrhenius plot, intercept is equal to: A. \(\dfrac{{{{\text{E}}_{\text{a}}}}}{{\text{R}}}\) B...
In Arrhenius plot, intercept is equal to:
A. REa
B. ln A
C. ln K
D. log10a
Solution
Arrhenius equation relates to the dependence of reaction rates on the temperature. The expression used to derive the intercept of the Arrhenius plot is k = Ae - Ea/RT. This expression is also known as Arrhenius equation. The plot is drawn in between the values of ln K and 1/T.
Complete step by step answer:
First, let us know about the Arrhenius equation. As mentioned above the equation can be written as:
k = Ae - Ea/RT
Now, as we know that in the Arrhenius equation k depicts the rate constant of the reaction. A is considered to be the pre-exponential factor, T is temperature, R is the universal gas constant, and is the activation energy used in the chemical reaction.
Here, we have to find the intercept of the equation. So, let us consider logarithm on both sides, then it can be written as:
lnK = ln(Ae - Ea/RT)
If we solve it further then it can be written as:
lnK = lnA - RTEa
Now, we will compare this equation with another equation, i.e. y = mx + c
In this equation m represents the slope, and c represents the intercept of the plot.
Thus, after the comparison of the equations, we get slope = -REa, and the intercept is equal to ln A.
We can see the plot of the dependence of reaction rate on the temperature which indicates the Arrhenius plot as shown:
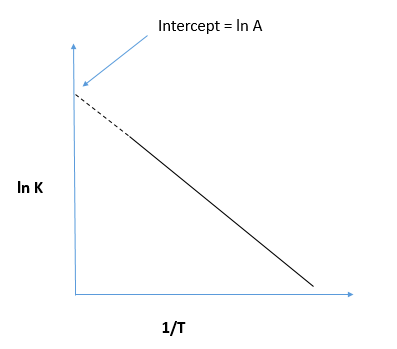
In the last we can conclude that in the Arrhenius plot, the intercept is equal to ln A.
Hence, the correct option is B.
Note: In this question, we have compared the Arrhenius equation with the equation representing straight lines. As mentioned, the graph between ln k, and 1/T is a straight line. According to the mathematical concept, the straight line equation depicts the slope, and intercept of the plot.
