Question
Question: In an intrinsic semiconductor, if \({N_e}\) is the number of electrons in the conduction band and \(...
In an intrinsic semiconductor, if Ne is the number of electrons in the conduction band and Np is the number of holes in the valence band then:
A) Ne>Np
B) Ne=Np
C) Ne<Np
D) None of the above
Solution
In an intrinsic semiconductor how many electrons become free, the same number of holes are created. Here the number of electron density is n and the number of hole density p is equal.
Complete step by step answer:
A semiconductor is a material that has a conductivity between conductor and insulator. Two types of semiconductors are here, one is a pure(intrinsic) semiconductor and another is an impure(extrinsic) semiconductor. Pure semiconductors are silicon (Si), germanium (Ge), etc. The intrinsic semiconductor number density of electron-hole pair is ni.
Let in a pure Silicon crystal the electron situated in the position of ‘A’ in the bond (fig-1) breaks the bond and goes to the position ‘X’. That means a valence electron becomes a conduction electron. In the meantime, in the position, ‘A’ there occurs a crisis of an electron, and then in the position, ‘A’ there generates a positive charge according to the corresponding electron clouds. This crisis of electrons in the bond is named Hole.
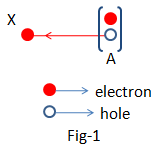
We can say, Ne=Np
Hence, the right option is in option (B).
Additional information:
Conductor:
The materials that allow the electric current to flow through them easily are called Conductors. The human body is a good example of good conductors. This property is called Conductivity.
Insulators:
The materials that hinder the flow of electricity are known as the Insulators. Wood and plastic are good examples of insulators. This property is called Insulation.
Note:
The mass-action law states that In thermal equilibrium, the product of the number density of electron n and the number density of hole p is constant. This constant is equal to the square of the number density of intrinsic semiconductor Ni.
That is, NeNp=( Ni)2
