Question
Question: In an experiment to determine the enthalpy of neutralization of sodium hydroxide with sulfuric acid,...
In an experiment to determine the enthalpy of neutralization of sodium hydroxide with sulfuric acid, 50cm3 of 0.4M sodium hydroxide were titrated thermometrically with 0.25M sulfuric acid. Which of the following plots gives the correct representation?
A.
B.
C.
D.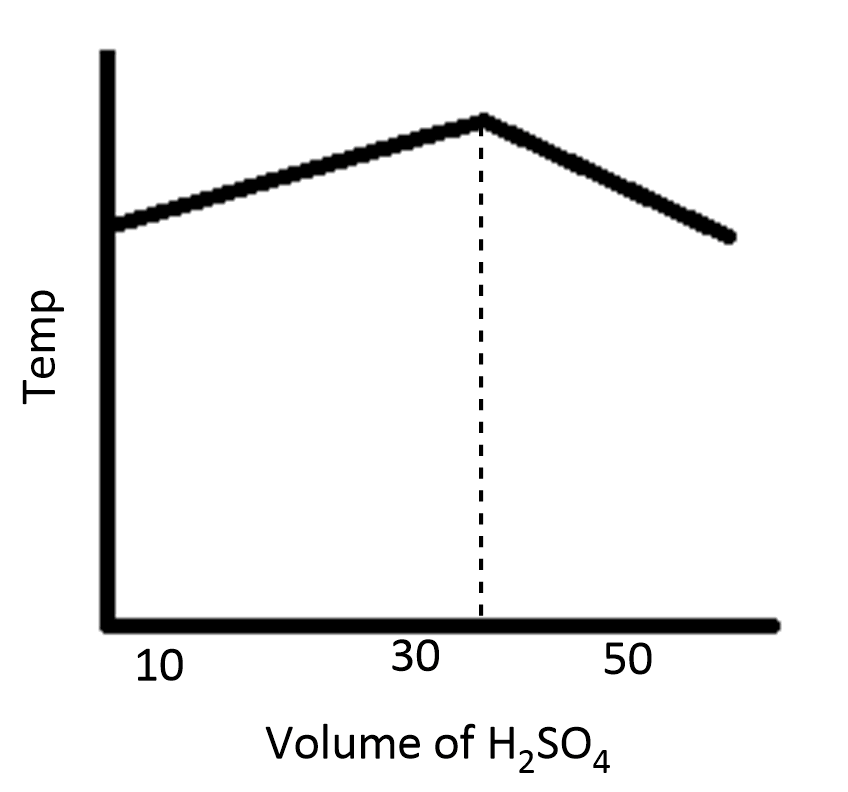
Solution
We can predict the correct representation by calculating the volume of the solution using the normality and the milliequivalents. Initially, we have to calculate the volume of the sulfuric acid solution by dividing the number of milliequivalents to the normality of the solution.
Complete step by step answer:
Volume of sodium hydroxide is 50cm3
Molarity of sodium hydroxide is 0.4M
Molarity of sulfuric acid is 0.25M
We have to know the number of milliequivalents of sulfuric acid.
So, the number of milliequivalents of sulfuric acid required for 20Meq of sodium hydroxide is twenty.
Let us consider the required volume of sulfuric acid to be V mL.
The volume of H2SO4 is calculated using the milliequivalents and volume. The product of normality and milliequivalents will be the volume.
We can write the formula as,
Number of milliequivalents=Normality×Volume
We know that the basicity of sulfuric acid is two.
So, we can calculate the volume by rearranging the equation of the number of milliequivalents.
Volume=NormalityNumber of milliequivalents
Let us now substitute the values of the number of mill equivalents and normality.
Volume=NormalityNumber of milliequivalents
On substituting the known values we get,
Volume=0.25×220
Volume=0.520
On simplification we get,
Volume=40mL
The volume of the solution is 40mL.
During the process of neutralization, the temperature increases and then decreases because of the increase in volume of the solution.
Therefore, the option D is correct.
Note: We have to know that Enthalpy of neutralization is always fixed in case of strong acid and a strong base: this is because strong acids and strong bases are fully ionized in dilute solution. The changes of enthalpy in neutralization are negative for a reaction of acid and base, and in this process heat is liberated out. In the case of weak acids or bases, the heat of neutralization is dependent on pH. In the absence of any added mineral acid or alkali a few amounts of heat is needed for complete dissociation to occur. The total heat evolved during the process neutralization would be smaller.
