Question
Question: In an experiment, m grams of a compound X (gas/liquid/solid) taken in a container is loaded in a bal...
In an experiment, m grams of a compound X (gas/liquid/solid) taken in a container is loaded in a balance as shown in figure I below. In the presence of a magnetic field, the pan with X is either deflected upwards (figure II), or deflected downwards (figure III), depending on the compound X. Identify the correct statement(s).
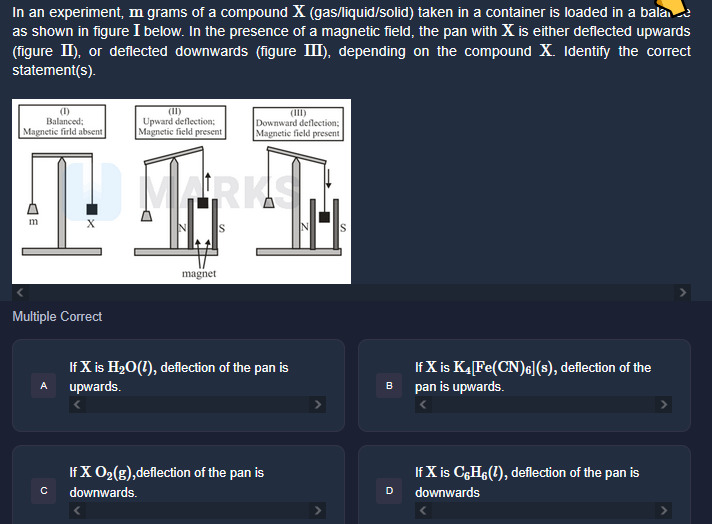
A
If X is H₂O(l), deflection of the pan is upwards.
B
If X is K₄Fe(CN)₆, deflection of the pan is upwards.
C
If X O₂(g), deflection of the pan is downwards.
D
If X is C₆H₆(l), deflection of the pan is downwards
Answer
A, B, C
Explanation
Solution
Concept:
- Diamagnetic substances are repelled by a magnetic field gradient; they lose effective weight (pan deflects upward).
- Paramagnetic substances are attracted to a magnetic field gradient; they gain effective weight (pan deflects downward).
Analysis of Options:
- H₂O(l): Water is diamagnetic → upward deflection. (Option A correct)
- K₄Fe(CN)₆: Ferrocyanide is diamagnetic → upward deflection. (Option B correct)
- O₂(g): Oxygen is paramagnetic → downward deflection. (Option C correct)
- C₆H₆(l): Benzene is diamagnetic → should deflect upward, not downward. (Option D incorrect)
Diamagnetic substances (H₂O, K₄[Fe(CN)₆]) are repelled by the magnet (upward deflection) while the paramagnetic substance (O₂) is attracted (downward deflection).
