Question
Question: In an acid-base titration, \(0.1M\) \(HCl\) solution was added to the \(NaOH\) solution of unknown s...
In an acid-base titration, 0.1M HCl solution was added to the NaOH solution of unknown strength. Which of the following correctly shows the change of pH of the titration mixture in this experiment?
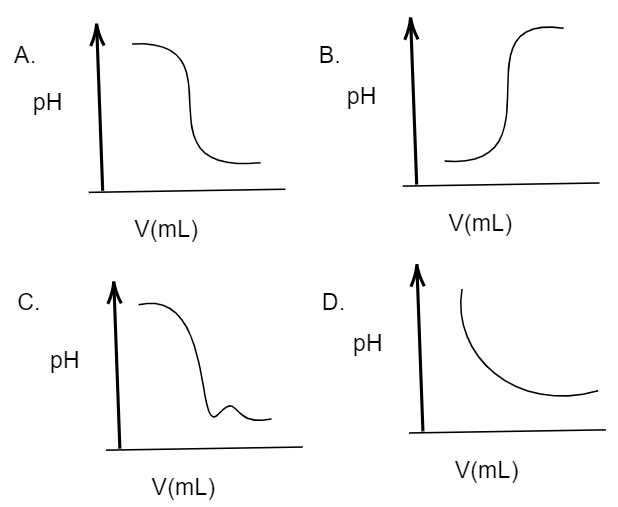
A. (A)
B. (C)
C. (D)
D. (B)
Solution
Remember the pH of an acidic solution shows lower value as compared to when a solution has a basic solution (i.e. will show higher pH value). So, first figure out what will be the pH of the first solution and whether it is acid or base and then find out what change in pH will happen when another solution (acidic or basic) is added.
Complete step by step solution:
Given that, In an acid-base titration, 0.1M HCl solution was added to the NaOH solution of unknown strength. So, here the solution containing HCl is acidic in nature, as we know HCl (hydrochloric acid) is a type of strong acid. Whereas the solution containing NaOH(sodium hydroxide, which is a strong base) will be basic in nature. We know that acidic solutions usually show a value of pH ranging from 0 to 7 while basic solutions will show a value of pH in pH scale ranging from 7 to 14. Further, it is said that the acidity decreases as the value of pH increases from 0 to 7, whereas the basicity decreases as the value of pH decreases from 14 to 7. So, here at first the solution has sodium hydroxide solution which is basic in nature, thus the pH will be high in the graph. And when hydrochloric acid (an acid) is added to this solution, the pH will obviously decrease indicating the consumption of the base and forming a salt. Thus, in the graph the pH value will decrease. So, the appropriate graph for this change should be:
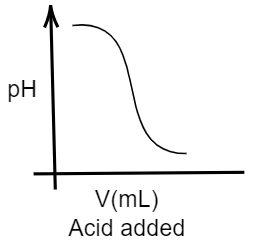
Hence, the correct option is A.
Note: You can get confused with the options given. Tally the right diagram with the correct option. The graph will have an increasing peak, when a basic solution will be added to an acidic solution indicating an increase in the pH, as acid is being consumed.
