Question
Question: In ammoniacal solution, dichromate in presence of H202 forms \({{\text{(N}{{\text{H}}_{\text{3}}}\te...
In ammoniacal solution, dichromate in presence of H202 forms (NH3)3CrO4 in which Cr is in IV the state. The structure is a pentagonal bipyramid. State true or false.
A) True
B) False
Solution
This (NH3)3CrO4 is a pentagonal bipyramidal structure. It involves the peroxo linkages. The chromium is tetravalent. It is synthesized from the dichromate in presence of 300/0 H2O2.
Complete answer:
It always mentioned that only a considerable number of peroxo-chromate complexes are known. These compounds are more or less stable and these peroxo compounds decompose slowly with the evolution of oxygen. Some of these peroxo compounds are explosive.
This (NH3)3CrO4 is synthesized by the treatment of alkaline dichromate solutions 300/0 H2O2. This forms the peroxo chromate(CrO83-). They are paramagnetic with the once unoccupied electron per formula unit.
We can say that in (CrO83-) the chromium is pentavalent.
When the mixture used for the preparation of (NH4)3CrO8 is heated at the 500C and cooled down to 00C a brown crystal of (NH4)3CrO4 are obtained-ray studies has revealed the structure of (NH3)3CrO4 as shown below:
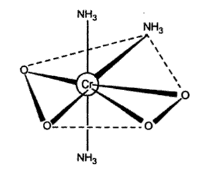
The (NH3)3CrO4 has the central metal atom as the chromium. The central chromium ion is surrounded by a total of seven ligands around it. The three are ammonia groups (NH3) and four of the oxygen are arranged around the central ion. The two superoxide ligands are bonded to the chromium. There are a total of five coordinating sites. For five ligands the possible structure is pentagonal bipyramidal since each oxygen of superoxide is considered as a single ligand.
Here, the oxidation state of chromium is a low valence state. The divalent chromium is coordinated to the two superoxide (-O-O-) ions. The compound contains the two unpaired electrons and thus chromium is diamagnetic. In other words, we can naturally consider a compound containing the tetravalent chromium IV which is coordinated peroxide ions.
Hence, when the ammoniacal solution, dichromate in presence of H2O2 forms (NH3)3CrO4 in which Cr Is in IV the state. The structure is a pentagonal bipyramid.
Hence, the statement is true. Correct option (A).
Note:
There are various peroxy –chromate for example deep blue chromium peroxide (CrO5), the blue peroxy-chromate Cr2O122- , and the red peroxy-chromate (CrO83-) .
