Question
Question: In allene (\({C_3}{H_4}\)), the type(s) of hybridization of the carbon atom is (are): A. \(s{p^2}\...
In allene (C3H4), the type(s) of hybridization of the carbon atom is (are):
A. sp2 and sp
B. sp2 and sp3
C. spand sp3
D. only sp2
Solution
The structures which have a double bond have sp2 hybridization and they have trigonal planar geometry. But if the structure has a triple bond equivalent in it with a linear structure, it has a sp hybridization. The sp3 hybridization has a tetrahedral geometry.
Complete answer:
Allene is an organic compound that comprises 3 carbon atoms attached to each other with the help of 3 double bonds. The structure of allene is as follows:
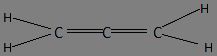
In the structure, the 1st and the 3rd carbon atoms have a trigonal planar geometry with bond angle of 120o in space. The two hydrogen atoms are connected to the 1st and 3rd carbon with the help of a σ−bond . Thus, the first and third carbon atoms have sp2hybridization.
Now, let us talk about the central carbon attached with the first and third carbon on both the sides. The central carbon has two σ−bondsand two π−bondsaltogether. Thus, it acts as a triple bond equivalent with 1 sigma bond and 2 pi-bonds. Thus, the central carbon atom has sp hybridization and the C=C=C bond is linear in shape.
Overall, allene has both sp2 and sp hybridization.
Thus, the correct option is A. sp2 and sp.
Note:
Allene is found naturally in various coloring pigments such as fucoxanthin and peridinin. They are primarily used as ligands in organometallic chemistry. It is also used as a catalyst in various organic synthesis reactions. In allenes, only one of the pairs of the double bonds is reducible.
