Question
Question: In adjoining P - V diagram, \[100J\] of heat is given in taking a system from A to C along the path ...
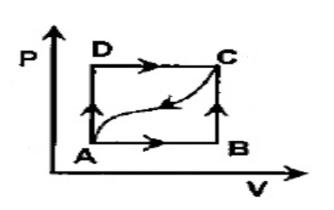
In adjoining P - V diagram, 100J of heat is given in taking a system from A to C along the path ADC and 50J of work is done by the system in this path. How much heat will be absorbed or given out if the work done on the system alone the cured path from C to A is 15J?
A) − 65 J.
B) − 75 J
C) − 95 J
D) − 115 J
Solution
A curve showing variation of volume of substance taken along the X axis and the variation of pressure taken along Y axis is called an indicator diagram.
Complete Step by step answer:
In the adjoining pressure and volume graph of an ideal gas is shown.
-The p-v diagram shows the feature of an idealized.
-The path between each state taking some process from A to D.
-The P –V diagram is taking the ABCD Path.
-From A to C along the path ADC, 100J of heat is given in taking a system.
-In this path 50J of work is done by the system.
-The work done on the system along the curved path from C to A is 15J
Therefore, the heat will be absorbed or given out value is −65J.
So, the correct answer is option (A).
Additional information:
-P-V diagram: On the left we have plotted the pressure versus the volume, which is called a p-V diagram.
-In a p-V diagram, from the upper left to the lower right, we say that the lines have a constant temperature curve.
-On this process were performed at the constant temperature is known as isothermal process.
Note: The p-v diagram is originally called indicator diagram.
-The pressure and volume diagram is used to describe corresponding changes in a system.
-The pressure and volume diagram is used to estimate the net work performed by a thermodynamic cycle.
-The total area under the curve on a PV diagram, is we can find the work done by determining.
