Question
Question: In acidic and alkaline solution amino acid exists as a- (A) Positive and negative ion respectively...
In acidic and alkaline solution amino acid exists as a-
(A) Positive and negative ion respectively
(B) Negative and positive ion respectively
(C) Neutral in both medium
(D) None of these.
Solution
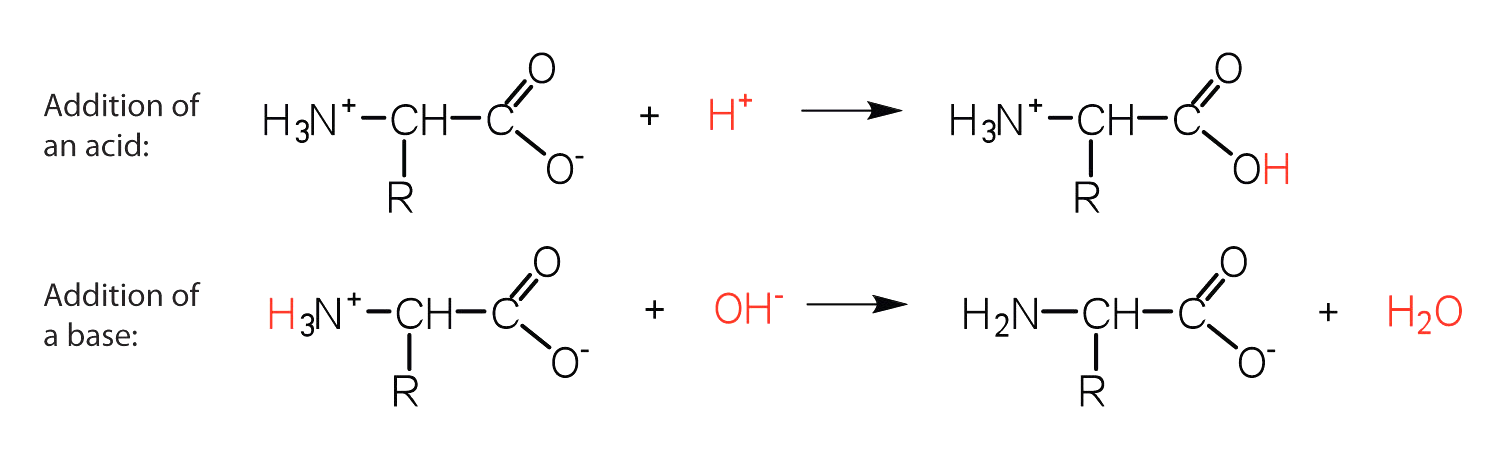
In acidic conditions, the amino acid acts as a base and accepts a proton at the amino group. In alkaline conditions, the amino acid acts as an acid and donates a proton from its carboxyl group.
Complete step by step solution:
-The structure of an amino acid allows it to act as both an acid and a base. This is because at a certain pH value (different for each amino acid) nearly all the amino acid molecules exist as zwitterions.
-If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion and the amino acid becomes positively charged.
-If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
In both circumstances, the amino acid acts to maintain the pH of the system- that is, to remove the added acid ( H+ ) or base ( OH− ) from solution.
-Thus, in acidic medium, the amino acid has a positive charge, thus a positive charge. In a basic medium, it possesses a negative charge.
Clearly, the answer is A.
Note: Zwitterion is a molecule that contains an equal number of positively- and negatively-charged functional groups. In an acidic and basic medium, it possesses a charge and becomes ions.
