Question
Question: In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is ...
In a thermodynamic process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by TΔX, where T is temperature of the system and ΔX is the infinitesimal change in a thermodynamic quantity X of the system. For a mole of monatomic ideal gas X=23Rln(TAT)+Rln(VAV). Here, R is gas constant, V is volume of gas, TAand VAare constants.
The List-I below gives some quantities involved in a process and List-II gives some possible values of these quantities.
| List I | List II |
|---|---|
| I. Work done by the system in process 1→2→3 | P. 31RT0ln2 |
| II. Change in internal energy in process 1→2→3 | Q. 31RT0 |
| III. Heat absorbed by the system in process 1→2→3 | R. RT0 |
| IV. Heat absorbed by the system in process 1→2 | S. 34RT0 |
| T. 31RT0(3+ln2) | |
| U. 65RT0 |
If this process on one mole of monatomic ideal gas is as shown in the TV-diagram with P0V0=31RT0.
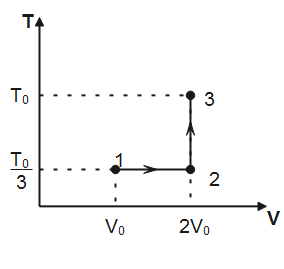
Which of the following is the correct match?
A. I→P,II→R,III→T,IV→S
B. I→P,II→T,III→Q,IV→T
C. I→P,II→R,III→T,IV→P
D. I→S,II→T,III→Q,IV→U
Solution
First analyze the type of process from the TV-diagram given. Then, calculate the work done, change in internal energy during each process and for the process 1→2→3 as well. First law of thermodynamics can be used to determine the heat transferred during any process.
Formula used: In an isothermal process, W=nRTlnViVf
In an isochoric process, ΔU=2fnRΔT
First law of thermodynamics, Q=ΔU+W
Complete step by step answer:
Note that, in process 1→2 temperature is constant hence, the process is isothermal. In process 2→3, volume of the gas remains conserved, therefore, the process 2→3 is isochoric. Degree of freedom for a monatomic gas, f=3
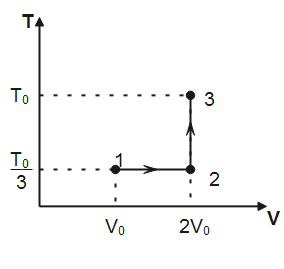
(I) Work done for isothermal process 1→2 is given by
W1→2=nRTlnViVf
Where n is the number of moles of the gas, T is the absolute temperature and Vf&Vi denotes final and initial volume of the gas respectively. Substituting the values observed from plot, we have
W1→2=1×R×3T0lnV02V0=3RT0ln2
Work done for the isochoric process 2→3 is zero. Since, no volume is changed during an isochoric process, the work done (which is linearly proportional to change in volume) is zero.
W2→3=0
Therefore, work done by the system in process 1→2→3 is
W1→2→3=W1→2+W2→3=3RT0ln2+0=3RT0ln2
This implies that I→P
(II) Internal energy changed in process 1→2→3 is given by
ΔU1→2→3=ΔU1→2+ΔU2→3
This implies that,
ΔU1→2→3=2fnRΔT1→2+2fnRΔT2→3
Substituting the values observed from the plot, we have
ΔU1→2→3=2f×1×R(3T0−3T0)+2f×1×R(T0−3T0)=23R×32T0
Solving the above equation, we obtain
ΔU1→2→3=RT0
This implies that II→R
(III) According to the first law of thermodynamics, heat transferred in a thermodynamic process is the sum of change in internal energy of the system and work done by the system. That is,
Q1→2→3=ΔU1→2→3+W1→2→3
Substituting the values, we obtained above, we have
Q1→2→3=RT0+3RT0ln2
Q1→2→3=3RT0(3+ln2)
This implies that III→T
(IV) Similarly, for process 1→2
Q1→2=ΔU1→2+W1→2
Substituting the values, we get
Q1→2=0+3RT0ln2=3RT0ln2
This implies that IV→P
Hence, option D is correct.
Note: In TV-diagram, line parallel to horizontal axis shows an isothermal process and line parallel to vertical axis shows an isochoric process. The work done in an isochoric process is zero.
According to the first law of thermodynamics, heat transferred in a thermodynamic process is the sum of change in internal energy of the system and work done by the system. This law is based on conservation of energy.
