Question
Question: In a plot of log k vs \(\dfrac{1}{T}\) the slope is: (a)- \(\dfrac{-{{E}_{a}}}{2.303}\) (b)- \(...
In a plot of log k vs T1 the slope is:
(a)- 2.303−Ea
(b)- 2.303REa
(c)- 2.303Ea
(d)- 2.303R−Ea
Solution
The equation called Arrhenius equation is usually written as k=Ae−Ea/RT where the pre-exponential factor A is a constant and is called frequency factor andEa is called the activation energy, R is the gas constant and T is the temperature. The activation energy is calculated by the formula logk1k2=2.303REa[T2T1T2−T1] where k1 and k2 are rate constants at different temperatures.
Complete step by step answer:
The Arrhenius equation is usually written ask=Ae−Ea/RT.
And its log form is:
logk1k2=2.303REa[T2T1T2−T1]
To test the validity of the Arrhenius equation, let us consider the equation as:
lnk=−RTEa+lnA
Or it can be converted into:
logk=−2.303RTEa+logA
This equation can be written in the form of the equation of the straight line.
The equation of the straight line is: y=mx + c
So, in the straight line equation, when we plot a graph between y and x, we get m as the slop.
Similarly when we plot the graph for the equation logk=−2.303RTEa+logA:
logk is the y and T1is the x, the validity of the equation is confirmed.
So, when we plot the graph we get m = 2.303R−Ea
So, the slope of the line = 2.303R−Ea
The graph of log k vs T1 is given below:
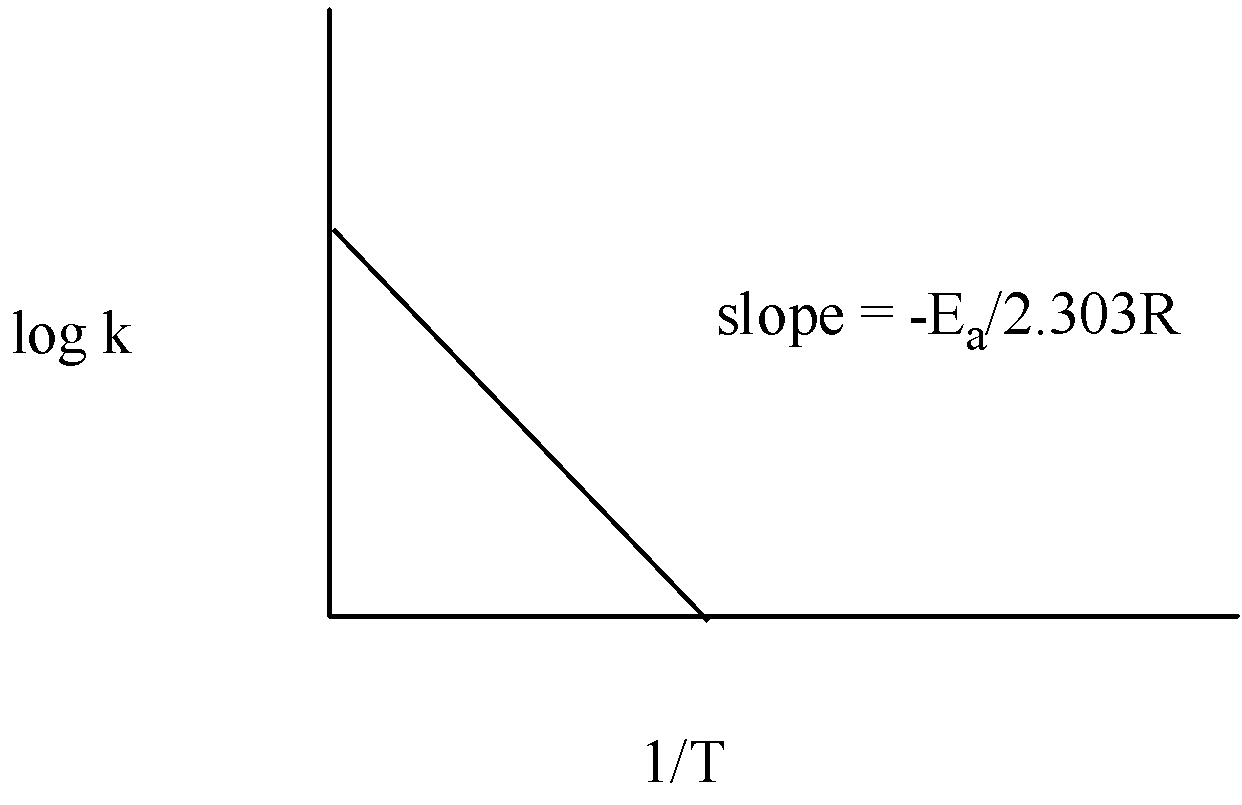
Thus, measuring the slope of the line, the value of Ea can be calculated.
So, the correct answer is “Option D”.
Note: If we plot a straight line graph for the equation lnk=−RTEa+lnA, the y will be ln k and the x will be T1 so in this case we get the slope of−REa. so, by converting any equation to the straight-line equation we can find the slope of the equation.
