Question
Question: In a photocell circuit the stopping potential,\[{{V}_{0}}\] is a measure of the maximum kinetic ener...
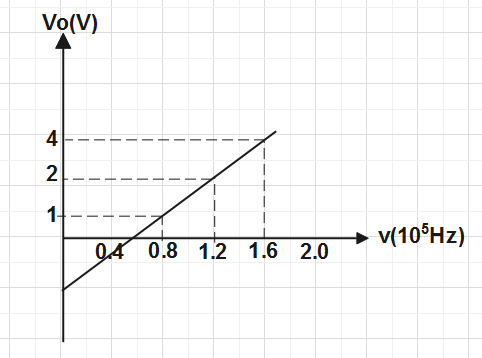
In a photocell circuit the stopping potential,V0 is a measure of the maximum kinetic energy of the photoelectrons. The following graph shows experimentally measured values of stopping potential versus frequency υ of incident light.
The values of Planck's constant and the work function as determined from the graph are (taking the magnitude of electric charge to bee=1.6×10−19C)
Solution
In this type of graphical question in which graph of Stopping potential and frequency is given then we have to use the slope of graph to find the value of planck's constant represented by h and using the intercept on y axis from the graph to find the work function of the metal surface which is represented by Wo .
Complete step-by-step solution:
According to Einstein’s photoelectric equation using the conservation of energy.
Energy of the incident photon= Maximum Kinetic energy of photoelectrons Work Function.
The maximum Kinetic energy of photoelectron is given by-
Kmax=hν−W0 (Equation 1)
If V0 is the stopping potential ,then
Kmax=eV0
∴eV0=hν−Wo
∴V0=(eh)ν−eWo (Equation 2)
Compare this equation with the straight line equation,
y=mx+c(Equation 3)
where m is the slope of the line and c is the intercept on the y-axis.
Compare these two equations 2 & Equation 3
We get,
Slope of graph m=eh
Intercept on y axis c=−eW0
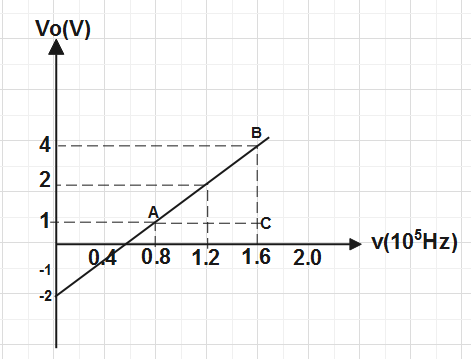
Now we have a consider a triangle ABC in the graph for calculating the slope of graph & intercept on
y- axis so that we are able to calculate the value of planck's constant and work function.
Slope of graph m=ACBC.
Now this slope will become equal to above slope and we get
eh=ACBC
⇒h=ACBC×e
Put the value of BC and AC from the graph and value of e is given in the question , we get⇒h=(1.6−0.8)×1015(4−1)×1.6×10−19
⇒h=0.8×10153×1.6×10−19
∴h=6×10−34Js
This is the required value of Planck’s constant.
Now we have to calculate the work function using the relation
Intercept on y axis c=eW0.
Here from this equation, we get
Work function is equal to e times the magnitude of the intercept on the vertical axis.
W0=ce
