Question
Question: In a laboratory setup for collecting a gas by water displacement, which of these gases could not be ...
In a laboratory setup for collecting a gas by water displacement, which of these gases could not be collected over H2O because of its solubility?
A) CO2
B) NO
C) O2
D) NH3
E) CH4
Solution
We must know that the gases have different solubilities in water. A number of the gases dissolved in water and provides new products by reaction. Whereas, some gases don't show this behaviour.
Complete step by step answer:
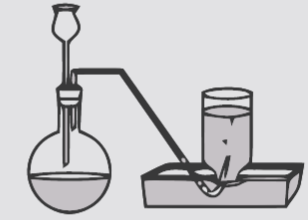
We must remember that the gases produced within the laboratory are collected by a water displacement method. To gather gas by this system the gases shouldn't dissolved in water.
In a laboratory setup for collecting a gas by water displacement, ammonia gas couldn't be collected over water due to its solubility. Ammonia can form hydrogen bonds with water due to which it's extremely soluble in water. For collecting a gas by water displacement through the laboratory set up, the gas to be collected should be water insoluble.
Therefore, the option D is correct.
The other compounds are easily soluble in water and hence they're collected over water by water displacement methods. Solubility of the substance depends upon the temperature, pressure, physical and chemical properties of the solute and solvent.
Hence option D is the correct answer.
Note:
In water displacement method, a beaker is crammed with water and it's kept the wrong way up during a beaker of water. A rubber glass tube on one end is attached to a reaction flask while the opposite end is kept under water within the beaker. The gas formed within the reaction flask, passes through the tube and displaces the water within the bottle. Once the gases are filled within the flask and it's fitted with a rubber cork. Since the gas is collected over water it's mixed with water vapors.
