Question
Question: In a given structure of acrolein, the CO bond is most polar, and O-atom is the most negative atom. I...
In a given structure of acrolein, the CO bond is most polar, and O-atom is the most negative atom. If true
enter 1, else enter O.
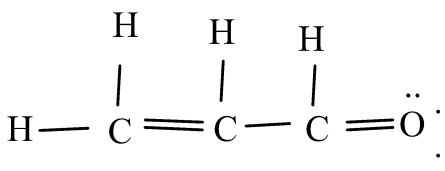
Solution
Propenal is another name to acrolein, it is an unsaturated aldehyde. Like every other aldehyde it goes under various reactions. It is found in fruits like walnut, papaya etc. it is yellow in color.
Complete step by step answer:
Let us understand the structure of acrolein, delocalization of pi-electrons takes place in acrolein towards the most electronegative atom. Delocalization occurs for those pi electrons where there is an adjacent sigma bond. In acrolein, oxygen is a most electronegative element and the delocalization of electron takes place toward oxygen due to C-O bond is most polar and O-atom is a most electronegative atom.
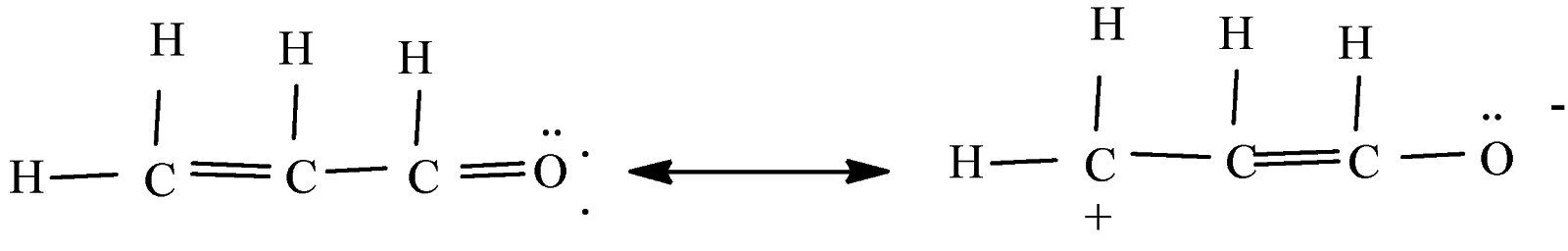
The bond between carbon and oxygen has a difference of 1.0 in electronegativity. In the C-O bond, the oxygen is oxidized and becomes a negative charge. Hence the C-O bond is most polar and O-atom is the most electronegative atom. Hence the statement is true and the answer is 1.
Acrolein is used to make plastics. This compound can be prepared by inserting a carbon monoxide molecule into the C-H bond of ethylene. The molecule with atoms bonded with different electronegativity. The dipole moment is used to measure the polarity of the molecule. The polarity of a molecule is measured in terms of dipole moment. The dipole moment for a polar molecule is non-zero and for a non-polar molecule dipole moment is zero.
Therefore the statement above is true hence we will enter1.
Note:
Be careful while handling acrolein as it is flammable, corrosive and acute toxic in nature. It is hazardous to the environment just like plastic as it is unsaturated.
