Question
Question: In a face-centered lattice with all the positions occupied by A atoms, the body-centered octahedral ...
In a face-centered lattice with all the positions occupied by A atoms, the body-centered octahedral holes in it are occupied by an atom B of an appropriate size for such a crystal. Predict the formula of the compound.
(A) AB
(B) A4B
(C) A4B3
(D) A4B5
Solution
To solve this question we first need to know what is a crystal cubic system. A crystal system in which the shape of the unit cell is a cube is known as a crystal cubic system. The three main types of these crystals are face-centered, body-centered, and primitive cubic.
Complete answer:
Now, in a body-centered cubic system (cl), there is one lattice point on the corner of each unit along with a lattice point at the center of the cube.
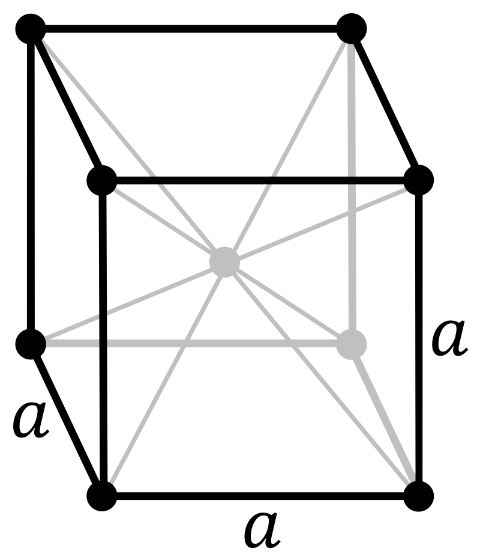
So the number of atoms in the unit cell will be
(81×8)+1=2
It has 6 net octahedral voids and 12 net tetrahedral voids.
In a face-centered cubic system (cF), there is one lattice point on the corner of each unit along with a lattice point at the center of the faces of the cube (which given 21 atom contribution).
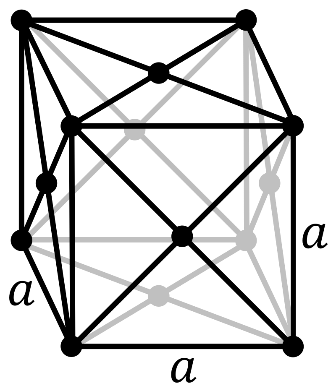
So, the number of atoms in the unit cell will be
(81×8)+(21×6)=4
It has 4 net octahedral voids and 4 net tetrahedral voids.
So, the number of atoms of A = 4 as it occupies lattice points in a face-centered unit.
And the number of atoms of B = 4 as it occupies octahedral voids in a body-centered unit.
So, the formula of the compound will be A4B4 or option (A) AB.
Note:
It should be noted that in a primitive cubic system (cP), there is one lattice point on the corner of each unit. Since the atom at the lattice point is shared by all the 8 adjacent sides of the cube.
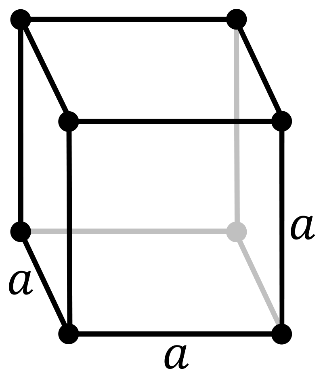
So, the number of atoms in the unit cell will be
81×8=1
It has a single cubic void in the center.
