Question
Question: In a cubic structure of diamond which is made from \(X\) and \(Y\) , where \(X\) atoms are at the co...
In a cubic structure of diamond which is made from X and Y , where X atoms are at the corners of the cube and Y at the face centres of the cube . The molecular formula of the compound is :
A: X2Y
B: X3Y
C: XY2
D: XY3
Solution
Whenever we are given a solid cube of a molecule , the positions of the substituent atoms determine the molecular formula of the compound. If the atom is in the edge centre it contributes $$$$$\dfrac{1}{4}totheparticularmolecule,ifitisinthefacecentreitcontributes\dfrac{1}{2}tothemoleculeandifitisinthecornerofthecubeitcontributes\dfrac{1}{8}$ to the molecule.
Complete step by step answer:
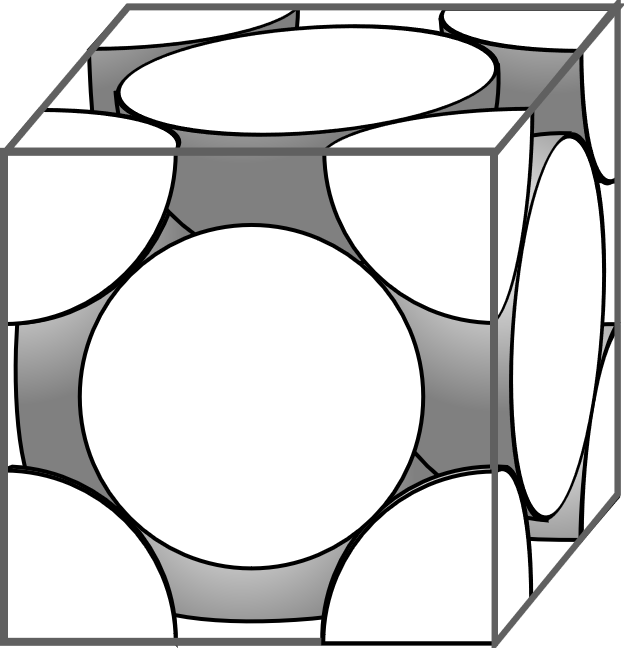
In the given question it is given that the X atoms are in the corners and the Y atoms are in the face centre. In a given cube there are eight corners and six faces. So there will be eight X atoms in the eight corners of the cube and six Y atoms in the six faces of the cube. The contribution of the corner atoms to a cube is 81 to the compound and the contribution of the face centred atoms to the compound is 21 . So the molecular formula of the compound will be
X81×8Y21×6=XY3
**So from a the above explanation and calculation it is clear that the correct answer of the question is
D: XY3 **
Additional information : When a cube has eight atoms in eight corners and one atom in the body centre it is known as body centred cube. When a cube has eight atoms in eight corners and six atoms in the six faces of the cube it is known as a face centred cube.
Note: Always remember that the atoms in the corner constitute 81 the compound , atoms in faces contributes 21 ,atoms in the edge centre contributes 41 and atoms in body centre contributes 1 to the compound. To find the molecular formula of the compound multiply the number of atoms in the particular positions with their respective contribution to the compound.
