Question
Question: In a certain experiment, three liquids were mixed. Five milliliters of methanol (\(C{{H}_{3}}OH\)), ...
In a certain experiment, three liquids were mixed. Five milliliters of methanol (CH3OH), five milliliters of hexane (CH4), and 5 milliliters of water (H2O) were added to a cylindrical tube. The tube was then sealed and shaken to mix the contents completely. After the liquid had settled, the test tube appeared as shown in the diagram. Methane and water formed a homogeneous mixture on the bottom of the test tube. The hexane formed a separate layer on top of this solution. Which of the following statements can be used to justify these observations?
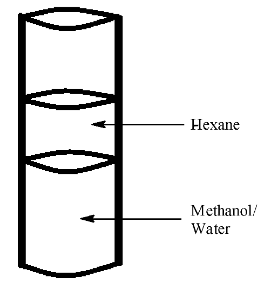
A. Methanol and water do not form favorable attractive forces with each other.
B. Hexane does not form favorable attractive forces with either water or methanol
C. Methanol, water, and hexane all form favorable attractive forces with each other
D. Hexane is less dense than either methanol or water so floats on top.
Solution
Hexane is immiscible in water, and no reaction or mixing takes place. Hexane has the density of 0.6 grams per litre.
Complete step-by-step answer: In order to answer our question, we'd like to grasp about azeotropes, still as positive and negative deviations. During this variety of deviations, the partial pressure of every component (say A and of solution is bigger than the pressure of course consistent with Raoult's law. These sorts of deviations are shown by the solutions during which solvent-solvent and solute-solute interactions are stronger than solvent-solute interactions. Since in solution, the interactions among molecules becomes weaker, therefore, their escaping tendency increases which ends within the increase in their partial vapour pressures. In such solutions total vapor pressure of the answer is additionally greater than the force per unit area required consistent with Raoult's law. For the answers with positive deviations there's an intermediate composition that the pressure level of the solution is maximum and hence, boiling point is that minimum. At this composition the answer distils at constant temperature without change in composition. an answer which distils without change in composition at a selected temperature is termed azeotrope or azeotropic mixture. The azeotrope in solutions with positive deviations is named minimum boiling azeotrope.
In negative deviations the partial pressure level of every component of solution is a smaller amount than the pressure needless to say in line with Raoult's law. This sort of deviations are shown by the solutions during which solvent-solvent and solute-solute interactions are weaker than solvent-solute interactions. Since in solution the interactions among molecules become stronger, their escaping tendency decreases which ends within the decrease in their partial vapour pressures. In such solutions total pressure level of the answer is additionally but the pressure level expected in step with Raoult's law. For the answers with negative deviations there's an intermediate composition that pressure of the solution is minimum and hence b.pt. is maximum. At this composition the answer distils at constant temperature without the change in composition. So it's an azeotrope. The azeotrope in solutions with negative deviation is termed maximum boiling azeotrope.
Now, allow us to come to our question. Hexane and water don’t mix with one another, and hexane is a smaller amount dense than water, hence it floats on top of the water.
So, we get the right answer as option D.
Note: An example of an answer with positive deviations, ie., an answer of n-hexane and ethanol, An example of an answer with negative deviation may be a solution of acetone and chloroform.
