Question
Question: In 3-methyl-2-cyclohexenone which hydrogen cannot undergo deuterium exchange when it reacts with \( ...
In 3-methyl-2-cyclohexenone which hydrogen cannot undergo deuterium exchange when it reacts with CH3OΘ/CH3OD ?
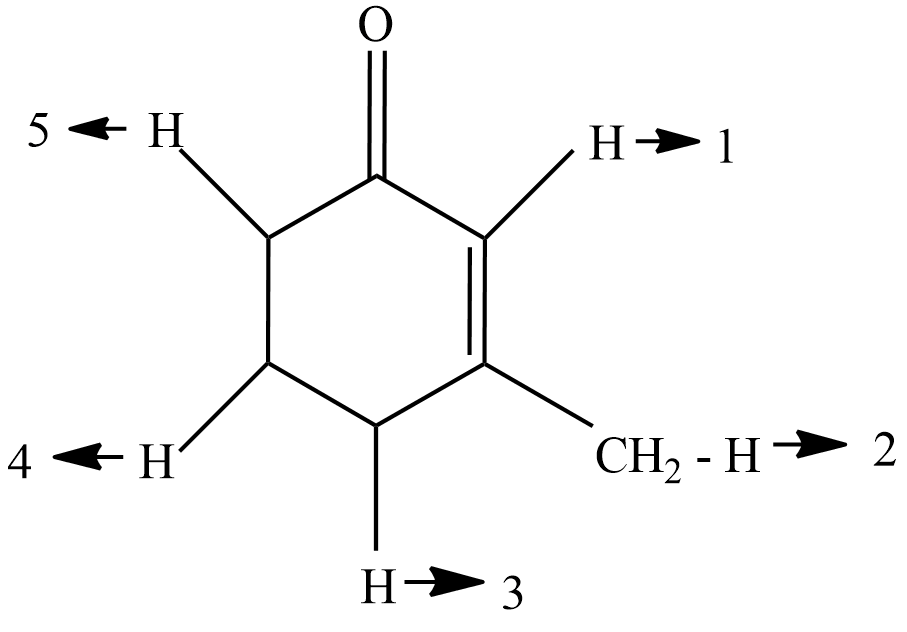
(A) H1,H4
(B) H4
(C) H3,H2
(D) H5,H3
Solution
Hint : This question talks abouts exchange of isotopes of Hydrogen. For this kind of question, we have to know about the Kinetic Isotopic effect in Tautomerism which says If we take a heavier isotope of Hydrogen then all the \alpha Hydrogen and the hydrogen which are in conjugation with the carbonyl group will be replaced by the heavier isotope of Hydrogen. So, in this question we will identify the \alpha Hydrogens and replace them with the heavier Isotope.
Complete Step By Step Answer:
Now, if we see in the diagram H1 and H5 are the alpha hydrogen because they are directly connected to the carbonyl group, so they will react with CH3OΘ/CH3OD they will get replaced. now H2 and H3 hydrogen are in conjugated hydrogen they will also get replaced when reacted with ch3 now we are left the H4 hydrogen if we see the diagram then it is clear that H4 hydrogen is neither a alpha hydrogen nor a conjugated hydrogen so it will not react with the CH3OΘ/CH3OD and will not be replaced.
The carbon atoms directly attached to the functional group are known as \alpha Carbon, Hydrogen atoms attached to \alpha carbon atoms are \alpha Hydrogen. Since they are directly attached to the \alpha carbon they become more reactive. So carefully observe the diagram to identify them.
The reaction taking place will be
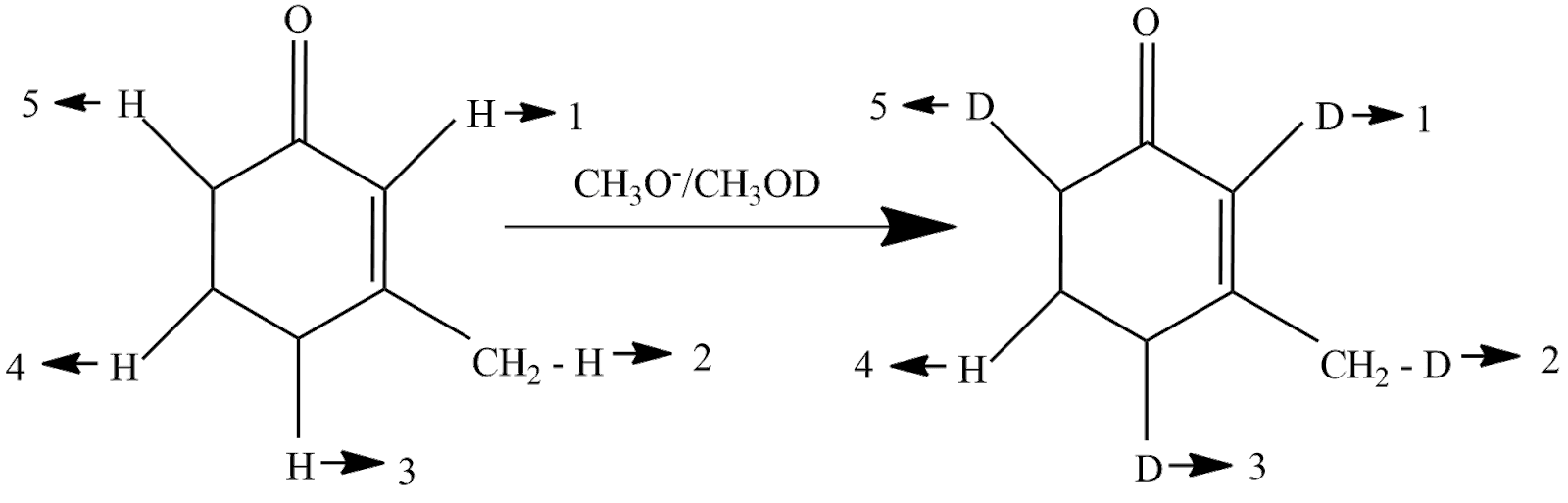
So, the answer is option (C).
Note :
Deuterium is an isotope of hydrogen also known as heavy Hydrogen with a nucleus containing one proton and one neutron so it has twice the mass of a normal hydrogen. When Deuterium is reacted with oxygen it forms D2O also known as Heavy Water.
