Question
Question: The thermochemical equation for the combustion of ethylene gas, $C_2H_4$, is $C_2H_4(g) + 3O_2(g) \...
The thermochemical equation for the combustion of ethylene gas, C2H4, is
C2H4(g)+3O2(g)⟶2CO2(g)+2H2O(l);
ΔHΘ=−337 kcal
Assuming 70% efficiency, calculate the weight of water at 20∘C that can be converted into steam at 100∘C by burning 1m3 of C2H4 gas measured at STP. The heat of vaporisation of water at 20∘C and 100∘C are 1.00 kcal kg−1 and 540 kcal kg−1 respectively.
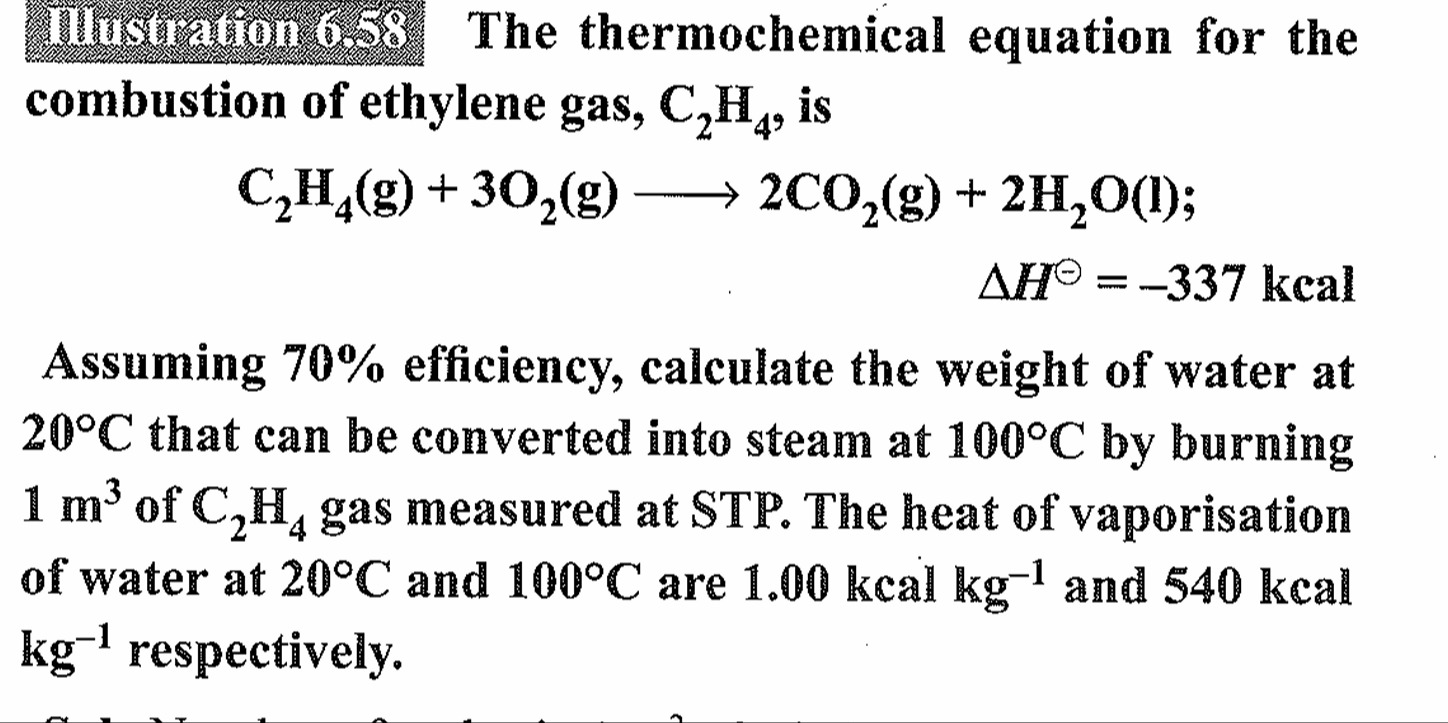
170 kg
Solution
The problem asks to calculate the mass of water that can be converted into steam by burning a given volume of ethylene gas, considering the efficiency of the process and the heat required for water's phase change and temperature increase.
1. Calculate the total heat released by the combustion of ethylene: The thermochemical equation for the combustion of ethylene is: C2H4(g)+3O2(g)⟶2CO2(g)+2H2O(l);ΔHΘ=−337 kcal This means that 1 mole of C2H4 releases 337 kcal of heat upon combustion.
First, determine the number of moles of C2H4 in 1m3 at STP. At STP (Standard Temperature and Pressure, 0∘C and 1 atm), 1 mole of any ideal gas occupies 22.4 L. Given volume of C2H4=1m3=1000 L. Number of moles of C2H4=Molar volume at STPVolume of C2H4=22.4 L/mol1000 L≈44.643 mol.
Total heat released (Qtotal) by burning 1m3 of C2H4: Qtotal=Moles of C2H4×Heat released per mole Qtotal=44.643 mol×337 kcal/mol=15049.251 kcal.
2. Calculate the useful heat available: The efficiency of the process is given as 70%. Useful heat (Quseful) = Qtotal×Efficiency Quseful=15049.251 kcal×0.70=10534.4757 kcal.
3. Calculate the heat required to convert water at 20∘C to steam at 100∘C per kg of water: This process involves two steps: a. Heating water from 20∘C to 100∘C: The problem states "heat of vaporisation of water at 20∘C and 100∘C are 1.00 kcal kg−1 and 540 kcal kg−1 respectively." The value 1.00 kcal kg−1 for heat of vaporization at 20∘C is physically incorrect (actual value is around 585 kcal kg−1). It is highly probable that 1.00 kcal kg−1 is intended to be the specific heat capacity of water (c = 1.00 \text{ kcal kg}^{-1} ^\circ C^{-1}), which is a common approximation. We will proceed with this interpretation. Heat required (Q1) = mass (m) × specific heat capacity (c) × temperature change (ΔT) For 1 kg of water: Q_1 = 1 \text{ kg} \times 1.00 \text{ kcal kg}^{-1} ^\circ C^{-1} \times (100^\circ C - 20^\circ C) Q1=1×1×80=80 kcal.
b. Vaporizing water at 100∘C: Heat required (Q2) = mass (m) × latent heat of vaporization (Lv) The latent heat of vaporization of water at 100∘C is given as 540 kcal kg−1. For 1 kg of water: Q2=1 kg×540 kcal kg−1=540 kcal.
Total heat required per kg of water (Qper_kg) = Q1+Q2=80 kcal+540 kcal=620 kcal/kg.
4. Calculate the weight (mass) of water that can be converted: Let mwater be the mass of water in kg. The useful heat available must be equal to the total heat required to convert mwater kg of water. Quseful=mwater×Qper_kg 10534.4757 kcal=mwater×620 kcal/kg mwater=620 kcal/kg10534.4757 kcal≈169.911 kg.
Rounding to a practical number of significant figures (e.g., three), the mass of water is approximately 170 kg.
The final answer is 170 kg.
Explanation of the solution:
- Calculate moles of C2H4 from its volume at STP (1m3=1000 L), using molar volume 22.4 L/mol.
- Multiply moles of C2H4 by its molar heat of combustion (337 kcal/mol) to get total heat released.
- Apply the 70% efficiency to find the useful heat available.
- Calculate the heat required to convert 1 kg of water from 20∘C to steam at 100∘C. This involves heating the water to 100∘C (using specific heat capacity, assumed 1 \text{ kcal kg}^{-1} ^\circ C^{-1}) and then vaporizing it at 100∘C (using latent heat of vaporization 540 kcal kg−1).
- Divide the useful heat available by the heat required per kg of water to find the total mass of water that can be converted.
