Question
Question: While the discharging of a lead storage battery following reaction take place. $PbO_2 + Pb + 4H^\op...
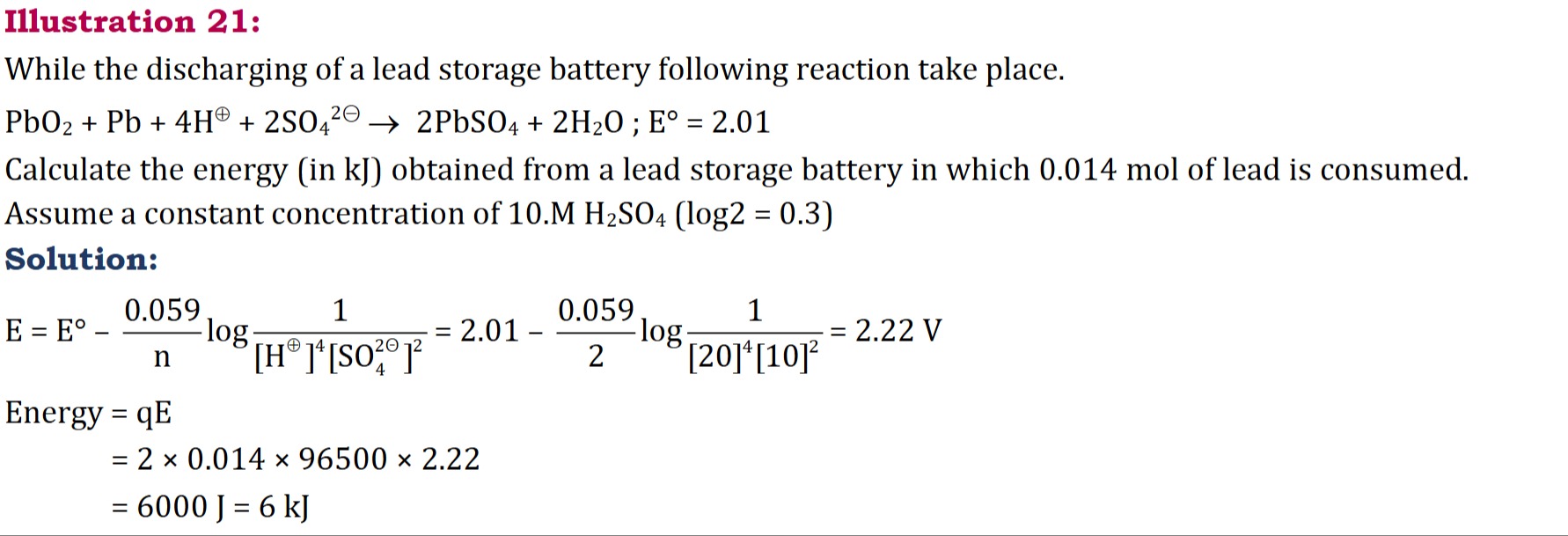
While the discharging of a lead storage battery following reaction take place.
PbO2+Pb+4H⊕+2SO42⊖⟶2PbSO4+2H2O ; E° = 2.01
Calculate the energy (in kJ) obtained from a lead storage battery in which 0.014 mol of lead is consumed.
Assume a constant concentration of 10.M H2SO4 (log2 = 0.3)
6 kJ
Solution
Step 1: Determine the number of electrons (n) transferred in the reaction.
The given reaction is:
PbO2+Pb+4H⊕+2SO42⊖⟶2PbSO4+2H2O
In this reaction, Lead (Pb) is oxidized from oxidation state 0 to +2 in PbSO4.
Pb⟶Pb2++2e−
Lead dioxide (PbO2) is reduced from oxidation state +4 to +2 in PbSO4.
PbO2+4H⊕+2e−⟶Pb2++2H2O
The number of electrons transferred (n) is 2.
Step 2: Calculate the concentrations of H⊕ and SO42⊖ ions.
Given that the concentration of H2SO4 is 10M. Sulfuric acid dissociates as:
H2SO4⟶2H⊕+SO42⊖
Therefore, [H⊕]=2×10M=20M
And [SO42⊖]=1×10M=10M
Step 3: Calculate the cell potential (E) using the Nernst equation.
The Nernst equation is:
E=E∘−n0.059logQ
For the given reaction, the reaction quotient Q is:
Q=[PbO2][Pb][H⊕]4[SO42⊖]2[PbSO4]2[H2O]2
Since Pb, PbO2, and PbSO4 are solids, their activities are taken as 1. The activity of water (solvent/product) is also taken as 1.
So, Q=[H⊕]4[SO42⊖]21
Substitute the values: E∘=2.01V, n=2, [H⊕]=20M, [SO42⊖]=10M.
E=2.01−20.059log[20]4[10]21
E=2.01−20.059log(20−4×10−2)
E=2.01−20.059log((2×10)−4×10−2)
E=2.01−20.059log(2−4×10−4×10−2)
E=2.01−20.059log(2−4×10−6)
E=2.01−20.059(−4log2−6log10)
Given log2=0.3 and log10=1.
E=2.01−20.059(−4×0.3−6×1)
E=2.01−20.059(−1.2−6)
E=2.01−20.059(−7.2)
E=2.01+0.059×3.6
E=2.01+0.2124
E=2.2224V≈2.22V
Step 4: Calculate the energy obtained.
The electrical energy obtained from a galvanic cell is given by:
Energy (Welec) = nmolFE
Where nmol is the total moles of electrons transferred, F is Faraday's constant (96500C/mol), and E is the cell potential.
Given that 0.014mol of lead (Pb) is consumed. From the stoichiometry of the reaction, 1 mole of Pb corresponds to the transfer of 2 moles of electrons.
So, the total moles of electrons transferred (nmol) = 0.014molPb×1molPb2mole−=0.028mole−
Energy = 0.028mol×96500C/mol×2.22V
Energy = 2702×2.22J
Energy = 5998.44J
Converting to kilojoules:
Energy ≈6000J=6kJ
Explanation of the solution:
-
Identify n: From the balanced reaction, 2 electrons are transferred (Pb goes from 0 to +2, PbO2 from +4 to +2).
-
Calculate ion concentrations: 10MH2SO4 yields 20MH⊕ and 10MSO42⊖.
-
Apply Nernst Equation: E=E∘−n0.059logQ.
Q=[H⊕]4[SO42⊖]21=[20]4[10]21.
Substitute values: E=2.01−20.059log(20−4×10−2)=2.01−20.059(−4log2−6log10).
Using log2=0.3, E=2.01−20.059(−4×0.3−6)=2.01−20.059(−7.2)=2.01+0.2124=2.22V.
-
Calculate Energy: Energy = nmolFE.
For 0.014mol of Pb, nmol=0.014×2=0.028mol electrons.
Energy = 0.028×96500×2.22=5998.44J≈6kJ.
