Question
Question: Calculate the cell e.m.f. and $\Delta G$ for the cell reaction at 298 K for the cell. Zn (s) | $Zn^...
Calculate the cell e.m.f. and ΔG for the cell reaction at 298 K for the cell.
Zn (s) | Zn2⊕ (0.0004M) || Cd2⊕ (0.2M) | Cd(s)
Given, EZn2⊕∣Zno = - 0.763 V; ECd2⊕∣Cdo = - 0.403 V at 298 K. F = 96500 C mol−1.
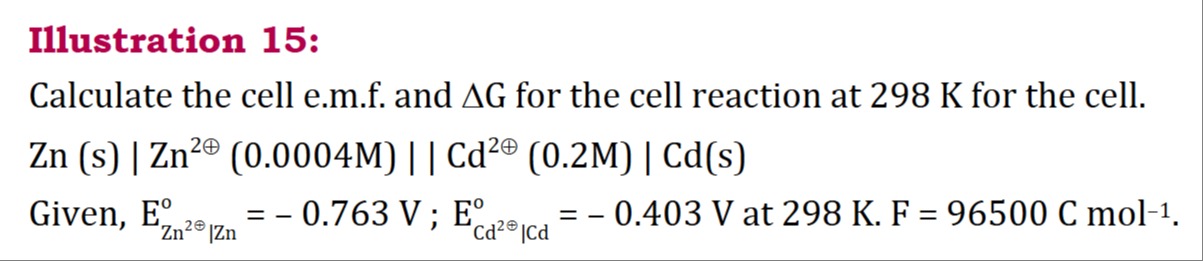
E_{cell} = 0.440 V, \Delta G = -84.90 kJ mol^{-1}
Solution
1. Identify Anode and Cathode, and write Half-Cell Reactions:
The given standard electrode potentials are: EZn2⊕∣Zno=−0.763 V ECd2⊕∣Cdo=−0.403 V
The species with the more negative standard reduction potential undergoes oxidation (anode). Comparing the two, Zn has a more negative potential than Cd. Therefore, Zn acts as the anode, and Cd2+ acts as the cathode.
Anode (Oxidation): Zn(s)→Zn2+(aq)+2e− Cathode (Reduction): Cd2+(aq)+2e−→Cd(s)
The overall cell reaction is: Zn(s)+Cd2+(aq)→Zn2+(aq)+Cd(s) From the overall reaction, the number of electrons transferred (n) is 2.
2. Calculate the Standard Cell Potential (Ecello): Ecello=Ecathodeo−Eanodeo Ecello=ECd2⊕∣Cdo−EZn2⊕∣Zno Ecello=(−0.403 V)−(−0.763 V) Ecello=−0.403 V+0.763 V Ecello=0.360 V
3. Calculate the Reaction Quotient (Q): For the cell reaction Zn(s)+Cd2+(aq)→Zn2+(aq)+Cd(s), the reaction quotient Q is: Q=[Cd2+][Zn2+] Given concentrations: [Zn2+]=0.0004 M and [Cd2+]=0.2 M Q=0.20.0004=2×10−14×10−4=2×10−3=0.002
4. Calculate the Cell e.m.f. (Ecell) using the Nernst Equation: The Nernst equation at 298 K (25°C) is: Ecell=Ecello−n0.0592log10Q Substitute the calculated values: Ecell=0.360 V−20.0592log10(0.002) Ecell=0.360 V−0.0296×log10(2×10−3) Ecell=0.360 V−0.0296×(log102+log1010−3) Ecell=0.360 V−0.0296×(0.3010−3) Ecell=0.360 V−0.0296×(−2.699) Ecell=0.360 V+0.0798904 V Ecell=0.4398904 V Rounding to three decimal places, Ecell=0.440 V.
5. Calculate the Gibbs Free Energy Change (ΔG): The relationship between Gibbs free energy change and cell potential is: ΔG=−nFEcell Where: n = 2 (number of electrons transferred) F = 96500 C mol−1 (Faraday constant) Ecell = 0.4398904 V (calculated cell e.m.f.)
ΔG=−2×96500 C mol−1×0.4398904 V ΔG=−84900.70768 J mol−1 Converting to kJ/mol: ΔG=−84.9007 kJ mol−1 Rounding to two decimal places, ΔG=−84.90 kJ mol−1.
