Question
Question: Illustrate with examples the limitations of Williamson’s synthesis for the preparation of certain ty...
Illustrate with examples the limitations of Williamson’s synthesis for the preparation of certain types of ethers.
Solution
The reaction was developed by Alexander Williamson in 1850. A few limitations of Williamson Ether Synthesis are tertiary alkyl halides or hindered primary or secondary alkyl halides undergo elimination in the presence of an alkoxide, this nucleophile also acts as a base.
Complete step by step answer:
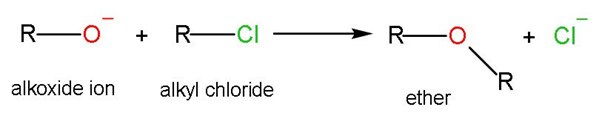
The Williamson ether synthesis is an organic reaction, forming an ether from a deprotonated alcohol (alkoxide) and organohalide. Reaction involves reactants as alkoxide ions with primary alkyl halide through SN2 or bimolecular nucleophilic substitution mechanism. This is a coupling reaction.
The general reaction mechanism is-
An example of this reaction is
[C2H5O]−[Na]++C2H5Cl→C2H5OC2H5+NaCl; where sodium ethoxide reacts with chloroethane to form diethyl ether.
-Limitations of Williamson’s synthesis:
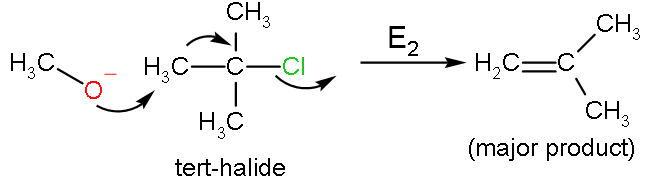
For SN2 reaction, there must be a good leaving group which is strongly electronegative mainly a halide. In the Williamson ether reaction, there is an alkoxide ion (RO−) which acts as the nucleophile, attacks the electrophilic carbon with leaving group, which is an alkyl tosylate. The leaving site should be a primary carbon, because secondary and tertiary carbon prefers an elimination reaction. This reaction does not favour the formation of bulky ethers such as di-tert butyl ether, due to steric hindrance and formation of alkenes is largely preferred.
Note:
The reaction between tert-butyl alcohol with primary halide is ether formation takes place, but primary alkoxide reacts with tert-butyl halide to do elimination.