Question
Question: Draw Resonance hybrid of the follo...
Draw Resonance hybrid of the follo
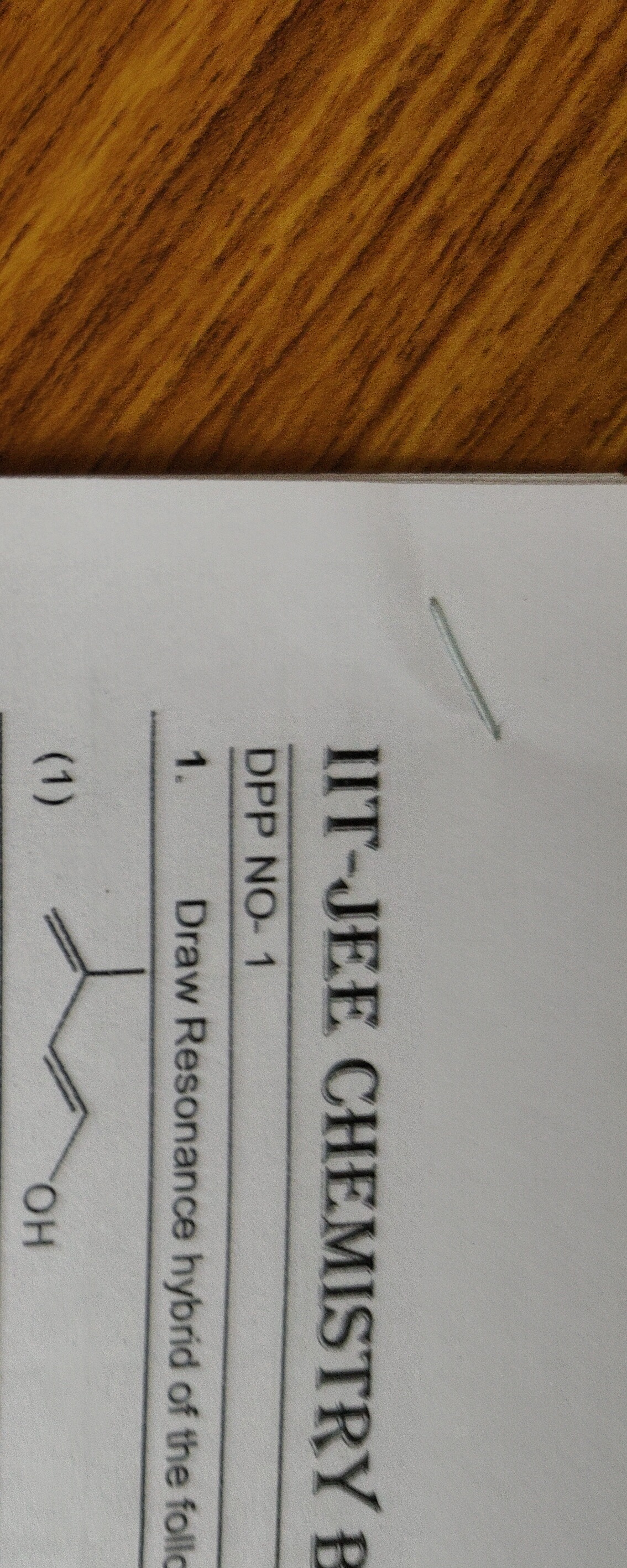
CH2=CH-CH2-OH
CH2-CH=CH-OH
CH2-CH-CH=OH
CH2=CH-CH2=OH
CH2=CH-CH2-OH
Solution
The given molecule is allyl alcohol, with the structure CH2=CH-CH2-OH. Resonance occurs when there is a delocalization of pi electrons and/or lone pairs within a molecule, leading to multiple contributing Lewis structures that are resonance structures. These structures are averaged to form the resonance hybrid.
For resonance to occur, there must be a conjugated system. A conjugated system typically involves alternating single and multiple bonds, or pi systems adjacent to lone pairs, charges, or empty p-orbitals.
In allyl alcohol (CH2=CH-CH2-OH):
- There is a pi bond between C1 and C2 (CH2=CH-).
- The oxygen atom of the hydroxyl group (-OH) has lone pairs.
- The carbon atom (C3) between the double bond and the oxygen atom is sp3 hybridized and saturated.
The sequence of atoms is C1=C2-C3-O. The pi bond is between C1 and C2. The lone pairs are on oxygen. The saturated carbon atom C3 separates the pi system of the double bond from the lone pairs on the oxygen atom. Therefore, there is no direct conjugation between the pi bond and the lone pairs of the hydroxyl group that would lead to significant resonance stabilization of the molecule itself.
Consequently, allyl alcohol does not exhibit resonance in the classical sense, unlike molecules like phenol or acetate ion. The electron delocalization that is characteristic of resonance is not present in the neutral allyl alcohol molecule.
In cases where no resonance occurs, the resonance hybrid is considered to be the molecule itself, as there are no other contributing resonance structures. Thus, the resonance hybrid of allyl alcohol is simply the structure of allyl alcohol.
